Physics or Fantasy
An Investigation of Modern Physics by Brian Williams-
Physics in the News- Gravity Waves. The Mechanics.
Posted on November 26th, 2015 No commentsWhat is a gravity wave? Stand on the sea shore and watch the tide come in. You are watching the result of a gravity wave. This gravity wave, twice daily(roughly) not only moves the seas but also moves the land.
The force of the moon’s gravity creates a moving force field (gravitational wave) that travels around the Earth. This wave is also affected by the gravitational force of the sun, the planets and, to an exceedingly small amount, all other stars and planets in the universe.
All bodies having mass and moving relative to another mass create a gravity wave.
Note that bodies that are in geosynchronous mutual orbits do not create a moving force field and therefore do not create a gravitational wave because the bodies do not move relative to each other.
Yes, I do understand that the current proposed experiments are an attempt by the physics establishment to force gravity into the ‘Wave Theory of Everything’ syndrome. Gravity is a force, not a wave. Light is particle transmission, not a wave.
However, they are attempting to use the same experimental set-up as used in the Michelson-Morley experiment, which is full of errors, both logical and mathematical.
In fact, the results obtained in the Michelson – Morley experiment actually proves that light does not travel at a constant velocity and that light is not a wave.
Also, the experiments are set up on Earth and therefore subject to massive gravitational forces relative to the forces that they are attempting to measure.
Latest World Headlines, gravity waves found, a few computer created graphics, two prints from graphs, but no evidence presented to allow the claims to be checked out.
Author – Brian Williams
See also;
How-Gravity-Works-and-What-Causes-It.
The Full Mathematics of the Michelson-Morley Experiment-Part-1
Liquid Balance On Saturn Satellite Mimas.
-
How to Build an Atom – The Mechanics.
Posted on August 27th, 2015 No comments“So far I have been unable to find any property of matter that cannot be explained by the use of only electrons and nuclei.”
———————————————————
The construction of an atomic model that satisfies all the requirements of scientific knowledge regarding both physical and biological facts must be our starting point. It must be able to explain colour, weight (mass), state (i.e. solid, gas or liquid), changes due to temperature, changes due to pressure, hardness, and softness, rigidity and flexibility, chemical reactions, toxicity, radioactivity, gravity, magnetism, and most importantly, life.
My theory explains all known properties of matter. (It therefore passes the requirements of a theory.)
I may be wrong, but certainly not as wrong as the Physics Establishment.
The reader will have problems due to fact that I go against all the current atomic hypotheses that he/she will have been taught or accepted. I can understand this because I had the same problem myself, many times over the years, finding it difficult to believe my own results. Eventually, it was easier to consider existing hypotheses only to pinpoint where the problems were. This was a case of selecting any particular hypothesis proposed by the physics establishment, assume it is wrong, and work out alternative hypotheses and then produce a working theory. 90% of current hypotheses cannot satisfy the title of theory because there are no explanations of how they could work.
One of the main reasons for physicists opting for high-speed electrons is to try to explain the energy of an atom. However, all energy is in one of two types/states, momentum or stress. (Note; In rotary motion the energy is stored in a combination of momentum and stress.) All atoms are in a state of stress. The reader may find this statement difficult to accept but it is true, whether you are considering chemistry, radiation or mechanics.
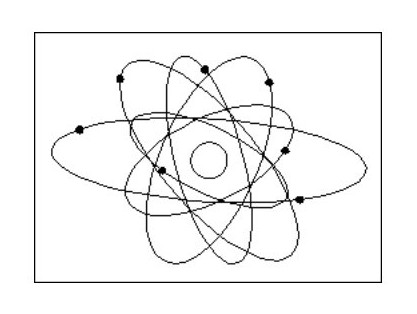
Fig. 1 below shows the image of the atom best known by the public.
Fig. 2
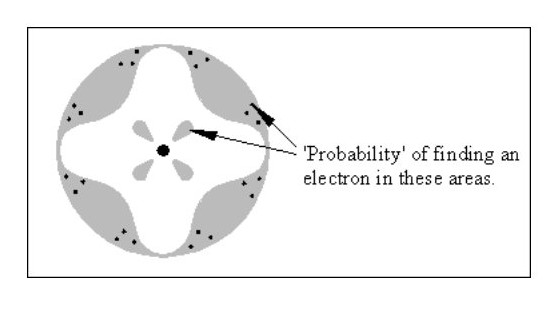 Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.
Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.Neither of these models is of any use in explaining the known properties of matter. How do two or more atoms form a molecule without all the electrons colliding? How can one atom be harder than another? How is one atom a liquid and another a solid?
Ask your local physicist any of these questions and he will mumble that it is too complicated to explain to mere mortals. The fact is that modern physics cannot explain any of them.
If electrons are not whizzing (whizzing is travelling at high speed) around causing dangerous collisions then it is easy to form molecules. All you have to do is to remove one electron from one atom and fill the gap by moving another atom so that one of its electrons fits in the gap in the first atom. Dead easy, you now have a molecule, two atoms jointed by a mutual electron. Both atoms have the right number of electrons therefore nothing has changed but we now have a molecule. However, life, physics and mechanics are not that simple.
Modern physics argues that the more electrons there are in an atom then the heavier it is. Therefore, as our molecule is an electron short of two atoms, it should be lighter than the original two atoms. Unfortunately, I totally disagree with this hypothesis because it is illogical.
The Structure of Atoms and the origin of Mass.
If Hydrogen is the lightest element and only has one atom, the one to one bond between the nucleus and the electron would make it far more difficult to remove this one electron from Hydrogen than trying to remove one electron from an atom with many electrons. (See Proton.) However, Hydrogen is the easiest element from which to obtain energy in the form of emitted electrons. In addition, Hydrogen has a ‘soft’ explosion indicating that the velocity of emitted electrons is relatively low. Although there are a few arguments (but not facts) against this, none of them overcomes the other problems of an atom with orbiting electrons.
If we reverse the hypothesis and say that the greater the number of electrons the lighter the atom, then this explains away many of the problems in physics without introducing any more. This would mean that the heaviest possible atom would have one electron.
It would also mean that the lightest detectable element, Hydrogen, would have the second largest number of electrons.
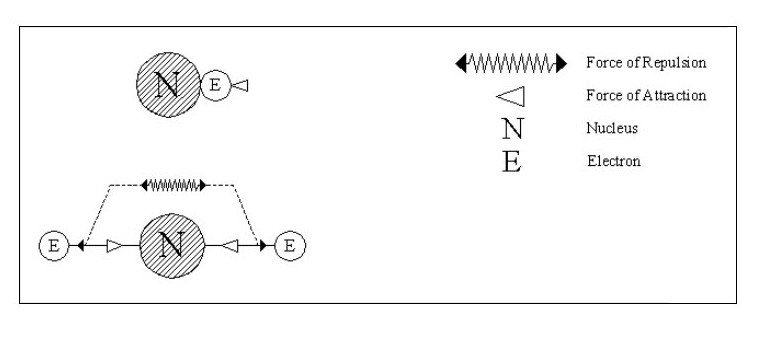
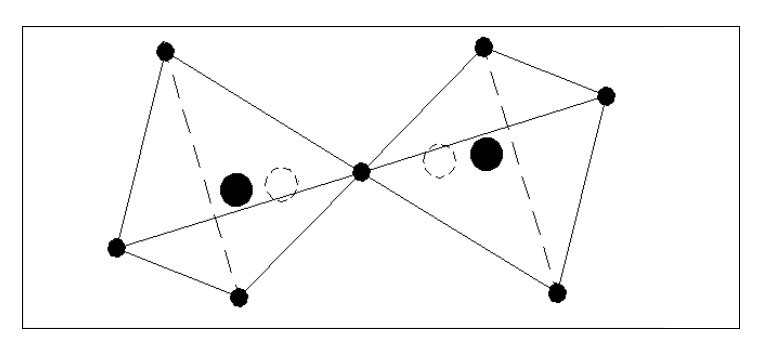
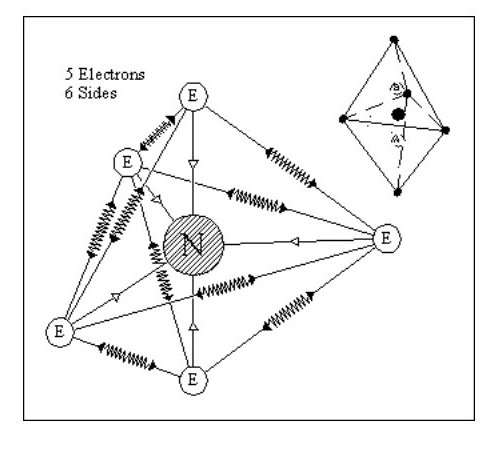
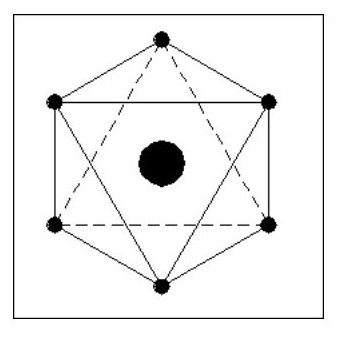
Let us consider some simple possible atoms. In Fig. 3, the top sketch shows a nucleus with a single electron. As this electron is not whizzing around it snuggles up against the nucleus due to the force of attraction. The lower sketch shows an atom with two electrons. In this case, the force of repulsion between the two electrons is acting against the force of attraction between the electrons and the nucleus. The electrons would align themselves on opposite sides of the nucleus. Although I have shown the electrons standing well clear of the nucleus, they could still be pressing against the nucleus but with less force. This would depend on the relative strengths of the attraction and repulsion forces.
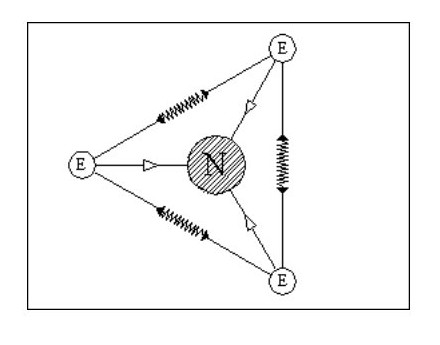
Let us add another electron. In Fig. 4, the three electrons have arranged themselves around the nucleus so that the forces of attraction and repulsion are balanced.
Now let me explain the problem that is irritating you. How can we have flat atoms? If the atom was a mile away from the nearest other atom or available electrons then Fig. 4 would be its natural state. In close proximity to other atoms, it would tend to combine with these atoms to form complex molecules, or try to accumulate more electrons.
It should be remembered that Fig. 4 is not a Lithium atom, it is an atom that is extremely heavy and one, like our previous two atoms, only being found at the core of collapsed stars or far out in space.
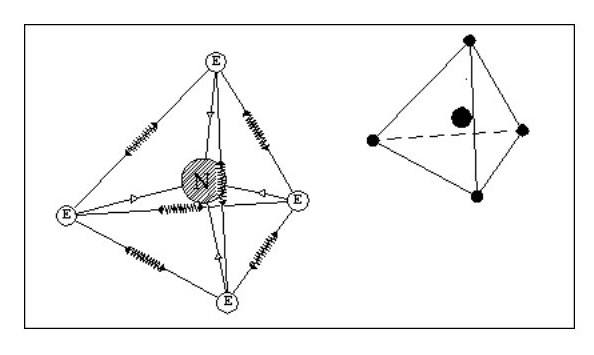
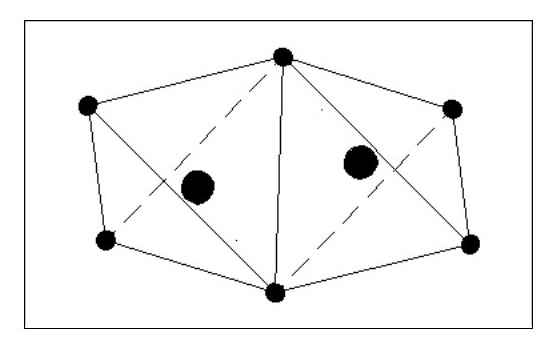
Let us now consider an atom with four electrons. Fig. 5 shows the atom in its ‘natural’ state.
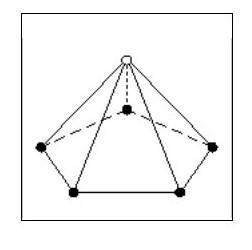
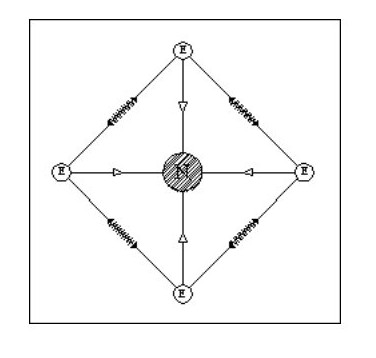 However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6
However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6Now that we have arrived at our first three-dimensional atom let us consider molecules. Using Fig. 6, we can create a simple molecule by using a single common electron.
With this type of molecular bond, it is obvious that the molecule will be quite flexible because one atom can flex and rotate relative to the other.
We now come to another deviation from modern physics. It is considered that for electrons to be attracted to the nucleus, the nucleus must have a positive charge (See Proton). Looking at the evidence from most experimentation would indicate that:
A. The nucleus is neutral and that the electron is attracted to the neutral state. Or
B The nucleus is attractive to both electrons and other nuclei, but the surrounding electrons reduce the affinity for other nuclei.
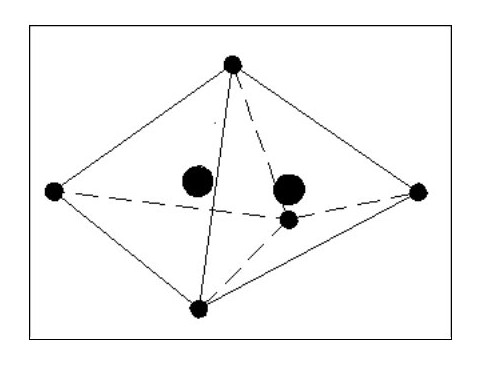
I feel that B. is the most logical. In the above sketch (Fig. 7) the shared electron presents a weakness in the surrounding shield of electrons, and the mutual attraction between the nuclei would pull them closer together as shown by the dotted circles. This in turn would distort the shapes of the two atoms as the forces balance. This weakness would be present in all molecules with similar results.
Let us now consider that these two atoms are joined as a molecule but by two common electrons instead of one. The formation of this molecule releases two electrons, therefore making the molecule heavier than the previous molecule. This new molecule is also less flexible than the previous one, one atom effectively being hinged off the other.
It should be considered that we are still dealing with extremely heavy atoms at this stage and the removal of electrons requires great pressure or a high energy.
If we now take our two atoms and create a molecule using 3 common electrons ( See Fig. 9 Below), we lose most of the flexibility and have an even heavier (and harder) molecule. It is clear that from two identical atoms we can produce the option of 3 different molecules each having different properties.
Now consider this point, if we split this molecule we are 3 electrons short of our original two atoms.
We could end up with one of our original atoms plus an atom with a single electron.
The modern physicist would argue that this result proves that the molecule was created by the addition of two totally different atoms. If it was difficult or impossible to split the molecule then the modern physicist would argue that this proves that the molecule is actually an atom!
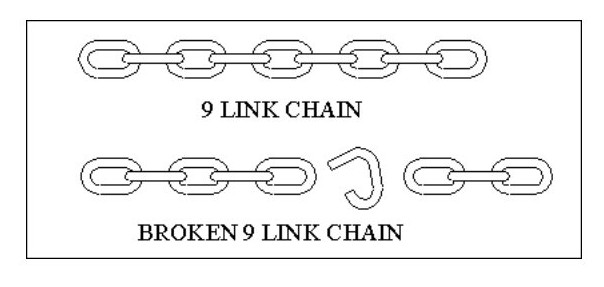
Most of our known substances considered to be atoms may in fact be difficult to split molecules, and many of our molecules may be conglomerations of identical atoms, but which when separated produce two or more totally different atoms. In the sketch below if the chain breaks as shown you could mistakenly argue that the 9 link chain is obviously created from a 5 link chain and a 3 link chain.
Let us now consider an atom with 5 electrons. We have a problem with this in that it is difficult to balance all the forces. On the other hand it gives us a glimmering of the reason for the life force.
Although similar in shape to Fig. 9 it only has one nucleus which can move between A and B with little effort, seriously distorting the shape of the atom in the process. This distortion would not destroy the atom, the overall forces being strong enough to retain its integrity.
It would take little effort to set up an oscillation in this atom. If an oscillation was set up and the atom shielded from all external forces, the oscillation could continue forever.
See Atomic Damping.
We are still looking at very heavy atoms, the weight/mass detectable being dependent on the number of electrons. The actual mass of an atom remains unchanged as this is the mass of the nucleus, which does not change. The electrons mask the affect of the nucleus’ mass. With a full complement of electrons the atom will have no detectable mass.
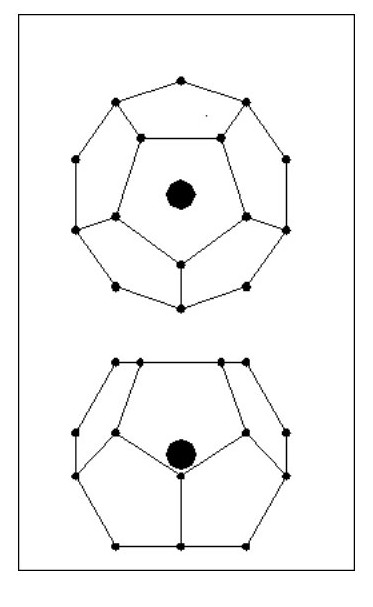
We will now look at a lighter atom. We will consider an atom in the shape of a Dodecahedron i.e. 12 sides and 20 electrons as Fig. 14.
(You might also like to look at The Origin of the Golden Section., because ratios relating to the dodecahedron tie in with golden section ratios.)
All the electrons lie on a sphere, equally spaced and all the same distance from the nucleus. Obviously 20 electrons will not mask the affect of the nucleus’ mass. [Note: With regard to the dodecahedron shape, this would clearly allow for molecular bonding with 1, 2 or 5 common electrons. However, under certain conditions 3 and 4 electron bonds could occur.]
Can we increase the numbers of electrons without causing problems?
If we add 12 electrons they will be attracted to the nucleus but will have problems with the repulsion forces of the existing electrons. These repulsion forces are weakest in the centres of the faces so the new electrons will align themselves in the centre of the faces.
But consider the following point: The existing electron sphere reduces the attraction forces for the new electrons and each new electron has to contend with the repulsion forces of 5 existing electrons. Our original dodecahedron will now have a five sided spike on each face as Fig. 15.
 It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.
It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.The new electrons will be equally spaced around the outer shell and will all be the same distance from the nucleus.
Now if we can have 12 electrons in a shell outside the dodecahedron, can we have a shell inside it containing 12 equally spaced electrons? The answer is yes. We can also have another shell on the outside containing 20 electrons, followed by further shells having alternatively 12 and 20 electrons.
The limit will be reached when the number of electrons completely mask the attraction force of the nucleus.
Interim Recap.
The orbiting electron hypothesis does not explain any of the properties of matter. See note.
- My theory explains rigidity and resilience.
- My theory so far gives a faint insight into the possible cause of the life force.
- My theory gives a logical cause for the crystal structure of many materials.
- My theory explains molecular bonding.
- My theory explains weight/mass. See note.
Note: The existing hypothesis states that the number of electrons governs the mass/weight of the atom. It does not explain how. The original reason for the statement that hydrogen had one electron was not based on any scientific reasoning or facts.
My readers may be wondering when I am going to explain about quarks, pi-mesons, etc. etc.
The answer is that there would be no point in wasting valuable space. The majority of these are hypothetical particles dreamed up to try and balance the faults in the existing weak hypotheses. Note; So far I have been unable to discover any property of matter that cannot be explained using only electrons and nuclei.
Let now consider an atom with six electrons. This would be in the form of 8 triangular faces.
As with the dodecahedron we could add an extra shell of electrons, in this case 8 of them. These electrons would would form a slightly distorted cube. If we add further shells they each would have 8 electrons until the forces blanketed the nucleus.
Now to answer your question as to how many electrons does it take to blanket the nucleus. I just don’t know. The lightest known substance is hydrogen. No-one knows how many electrons each atom of hydrogen has despite the physicists statement that it has only one.
The problem is that we really don’t know how many electrons any atom has. None of the experiments in the last 100 years has produced any logical figures and the standard tables of the elements can only be considered as pure guesswork.
We will take an arbitrary figure of 100 as the number of electrons required to blanket the nucleus.
Logically it would only take one additional electron to finally trip an atom into a condition that would make it undetectable. In the above atom the electrons are added in batches of 8. In the dodecahedron shaped atom the electrons are added in batches of 12 or 20. Obviously both families of atoms could get to the stage of being undetectable. It would appear to be unlikely that in either case would the final shell would need a full complement of electrons before the atom became undetectable.
However it just may require a full complement or at least a balanced part complement.
The Structure and Mechanics of Atoms and Mousetrap molecules
Let us consider chemistry and chemical reactions. Chemistry relates to the formation of molecules.
If you put powdered copper, zinc, carbon, titanium plus water into a tray and mix them, once they have settled you will still have powdered copper, zinc, carbon, titanium plus water in the tray. No new molecules will have been created. Why not?
Cu + C + Zn + Ti + H20 = ?????
You cannot get an answer for the above equation because it does not allow for the two most important variables; Temperature and pressure. (I’ve never come across a valid formula for a cup of tea either.)
Without variables no formulae are valid.
Under ‘natural’ conditions certain molecules can be created. Obviously ‘natural’ conditions vary depending on where you are. Natural conditions at the North Pole are different than natural conditions in the Sahara Desert. ‘Natural’ conditions on the Moon are different conditions than on Pluto or Mercury. Under ‘natural’ conditions many molecules cannot be created i.e. they need high /low pressure or high/low temperature or a combination.
Under ‘non-natural’ conditions many molecules can be created.
Most life on Earth evolved to suit gradually changing ‘natural’ conditions. Occasionally, unnatural conditions, such as volcanic activity occur that release molecules that have been created under unnatural conditions such as high temperatures and high pressures. Many of these molecules are toxic to most forms of life on Earth. Some lifeforms, such as the sea creatures around volcanic vents on the ocean floors, have evolved to handle the toxins, high pressure and high temperatures. To these creatures these conditions are now ‘natural’.
Under higher pressures atoms contract and under lower pressures atoms expand. If the pressure is high enough electrons are ejected from atoms (Exothermic reaction). THEREFORE they become different atoms that have qualities different from the original atoms.
Lighter atoms compress more than heavy atoms, therefore they will lose electrons easier than heavy atoms.
The gas Nitrogen has a completely different atom structure than liquid nitrogen.
Carbon and diamond have different atomic structures.
Water and Ice have different atomic structures.
If you place two dissimilar atoms that, (under normal conditions would not form a molecule), under increasing pressure you will arrive at condition such that the lighter atom would be able to form multiple electron bonds (MEBs) with the heavier one.
When this happens the molecule would be stable at this particular pressure. However, if the pressure is released, the molecule will either split into two separate atoms again or will remain as a molecule but with the lighter atom being in a distorted and stressed condition. This would make it vulnerable to forming MEBs with other atoms that it would not be possible under normal conditions.
Mousetrap Molecules
In affect, the molecule could become a ‘mousetrap molecule’ that could be easily triggered into forming strange/unnatural molecules or atoms, or even molecular explosions. Apart from toxins this would explain ‘radio activity’ and inflammability.
Radioactivity depends on a source of materials that have been subject to high pressures. Most of these are relatively stable in that only a few molecules per/second explode in any given mass. However, when they do the lighter atom disintegrates at high speed. Impurities in the ore tend to restrict the amount of radioactivity. Purifying the ore increases the ratio of mousetrap molecules until they start to trigger each other and you get a sustained reaction
————————————–
NOTE; Free Electrons have a very important role in chemistry. Free electrons are those not locked into the structure of an atom. They may be attracted to nearby atoms but can be easily moved around without affecting the structure of the atoms. Free electrons will still have a blanketing/insulating effect on the nucleus of the atom that would hide its true mass.
(Structural Electrons are those electrons that are part of the structure of atoms.)
Heat and Temperature.
Let me make the following statement: ATOMS DO NOT HAVE A TEMPERATURE.
Any isolated atom is completely devoid of any sense of temperature, it is neither hot, warm or cold.
If it loses an electron then this electron MAY be detected as heat.
Under pressure, atoms will reach a stage when the atomic structure starts to break down and electrons are emitted.
Temperature measurement in this case is dependent on its emission velocity.
——————————————————-
The mass of an atom is a force.
Let me apologise for the title of this post. You cannot build an atom. You can only disassemble an atom.
I was going to change the title but could not think of a better one.
———————————————-
Families of Atoms
MORE TO COME SHORTLY
Author; Brian Williams
Comments accepted.
-
Individuals names only, no company names.
-
Only one URL allowed.
-
Note that comments are now processed through a
separate computer.
Comments automatically deleted. (I do not see them.)
-
All comments from pornographic sites.
-
All comments from sales sites.
-
All comments not relevant to my website.
-
Tin, BPA, BPS and Phthalates.
Posted on July 19th, 2015 No commentsA Preliminary Investigation into My Death. See also “boil or carbuncle” .
At the present time I am dying due to continuous carbuncles that are effecting the operation of my normal bodily processes. In the last eight years there has rarely been periods of longer than 5 to 6 weeks that I have not been suffering from an active carbuncle.
At present (14th July 2015) I am suffering from a carbuncle in an awkward position on by backside. This is in its (I hope) final stage after 5 weeks continuous leaking. (Update; 10th September 2015. I am still having to change dressings twice a day, so no improvement yet.)
The medical profession claim these carbuncles are boils and insist on prescribing antibiotics that have no effect on the problems.
They claim that boils and carbuncles (and furunculea) are all the same thing. THEY ARE NOT. See “boil or carbuncle“.
60 years ago I learned that carbuncles are not caused by streptococci. Boils are caused by streptococci. This was a simple experiment. Having had a carbuncle two years before, when I had a painful, throbbing boil erupt on my neck I decided to try stannous oxide tablets, as these had an almost miraculous healing effect on the earlier carbuncle after months of injections and capsules of antibiotics provided by the medical ‘experts’. Stannous oxide tablets had no effect on the boil. Chemists knew there was a difference between carbuncles and boils but the medical profession did not and still refuse to accept that there is a difference.
Is this criminal negligence by the medical profession? In one way it is. The refusal of (or inability) of medical personnel to listen to patients is a serious problem. I have never had any doctor ask me whether the prescriptions he issued to me actually worked.
Some prescription medicines include questions about side effects. Is the fact that the medicine prescribed has no effect on the complaint or illness and therefore extends the duration of the problem, not a side effect?
I have recently obtained 20 grammes of Tin that I trying to purify sufficiently to pass my own safety requirements. This will probably take a few weeks. Note; I will still have the problem of determining whether the tin or the oxide is the crucial component: I feel that the oxide is the most likely but I hope that my body can create the oxide.
Update 6th April 2016. I am convinced that my body can handle handle the tin and use it for my benefit, however, I feel that tin oxide (Sn(ll) itself is providing the improvement and that oxygen in the blood is modifying a small proportion of the tin into SnO. Tin metal itself is not a natural substance.
******************************
Ongoing notes relating my current attempt to self medicate.
- At present (4th October 2015). Over the last two weeks I have been taking approximately 200 milligrams each day of tin powder. (Note this is now correct, there was a fault on my electronic weighing unit)
- Over this period I have noted the following; Swellings under both my arms have almost disappeared (after about 8 years). Pubic and underarm hair growth has increased. (But I am still going bald.) Over the last three days leakage from my current carbuncle has almost stopped. PLUS (relating to type 2 diabetes) sensitivity on the soles of my feet has improved, making walking a lot easier. It is obviously too early to get excited about these improvements. I will continue with the low dosage because I am still not confident about the quantity of contaminants. However, it does seem that by my body can use the tin itself.
- From the 1st of October 2015 I have rather complicated this series of tests by stopping taking statins. This was because it has been claimed by many sources that statins can cause leg muscle problems, which I have suffered from for about 18 months. Over the last two weeks I have noticed a significant improvement in my walking ability. A further unexpected result is a complete stoppage to my falling asleep at odd times during the day, often for 2 – 3 hours. I have not fallen asleep even once during the day since the 1st October.
- 1st December. Still not dosing off during the day. Carbuncle leakage stopped. No further improvement in walking.
Brian,
*****************************
It is only recently that I have looked into the background to the changeover to plastic lined tins. I naturally assumed that it was just to make more profits for the involved companies. However, I find that not only that the companies have effectively prevented access to a highly beneficial vital mineral (SnO), they have replaced it with a toxic alternative.
—————————————–
NOTE: I have used lots of information from the ACU-CELL website (http://www.acu-cell.com/) which I have found interesting, well written and logical. However, I have still not found their address so that I can write to them.
“Health Benefits & Toxicity of the Element Tin, and its Effect on Adrenals, Depression and Fatigue. Copyright © 2000-2015 Acu-Cell – Tin – Health Effects.
While Tin (Sn) has been established to be an essential trace element for some animals (they won’t grow well without it), some researchers are still unsure of whether tin is essential to human health. Daily dietary intake of tin from various food sources is in the 1 – 3 mg range, which is less than 1/10th of the daily intake obtained years ago before lacquering tin cans, switching to aluminum cans, or, in the more distant past, when tin cups or tin pans were still in use. Since bronze contains copper and tin, the use of tin has been established well past the Bronze Age, several thousand years ago.Rat studies have shown that tin-deficient diets resulted in poor growth, reduced feeding efficiency, hearing loss, and bilateral (male pattern) hair loss.”
“Tipton and Shafer examined tin in human tissue after accidental deaths.
They noted that tin was found in the aorta, brain, heart, kidney, liver, muscle, ovary, spleen, pancreas, testes, stomach, and uterus, but none was found in the thyroid of any victim, while the prostate, which usually shows no other trace element, had tin.”
[Note by Brian; Since the canning industry stopped the use of tin only and replacing the tin with a BPA lining I have had Pancreitus , Heart problems including a triple bypass and Prostate cancer.]
“Average concentrations were the same range as cobalt, iodine, chromium, and selenium, which are known vital nutrients. Inorganic tin is capable of entering into biological activity at saline pH, and it is far less toxic than other known vital trace elements such as copper and cobalt. In addition, tin levels do not vary statistically with age, gender, or geographical areas. Misk found traces of tin in the fetal heart and spleen, and higher levels in the liver, while Schroeder and others reported no tin in stillborns.”
Tin is associated with Iodine the same way as calcium is associated with magnesium (see “Tin & Iodine” for details). Tin supports the adrenal glands, and iodine supports the thyroid gland, with both subsequently affecting cardiac output: Tin + adrenals control the left side, and iodine + thyroid control the right side. In addition to low Vitamin C and/or Vitamin B1, low tin is a common nutritional cause of low adrenals, which can lead to left-sided cardiac insufficiency. While fatigue or depression may be experienced with cardiac insufficiency of either side, breathing difficulties or asthma are more common with left-sided cardiac insufficiency, and swelling of hands and feet is more common with right-sided cardiac insufficiency,regardless of the cause.Comparing thousands of patient records showed that better than 90% of patients tested exhibited moderately low, to very low levels of Tin when referenced to the status of all other essential trace minerals, making tin the most deficient element compared to any other trace mineral measured.I had 285 individuals taking part in the Nutritional evaluation of Tin, some on a short-term basis (3 weeks),and others on a long-term basis (1 – 2+ years), resulting in some valuable feedback on various responses encountered, including side effects, although the rather poor absorption of stannous oxide was a limiting factor in being able to achieve optimal cellular levels of tin in all subjects.Of the changes experienced after supplementing tin, negative reactions, e.g. stomach / digestive upsets, or skin reactions, were at par or less compared to the best tolerated trace minerals such as chromium, calcium, or magnesium. Positive health effects were numerous and included improvements with fatigue, some forms of depression, and a general increase in energy, well-being, and mood. There were also benefits with certain types of headaches, insomnia, asthma, or improvements with digestion, skin, or various aches and pains.Tin poisoning (https://en.wikipedia.org/wiki/Tin_poisoning)
Main article: Tin poisoning
Tin poisoning refers to the toxic effects of tin and its compounds. Cases of poisoning from tin metal, its oxides, and its salts are “almost unknown”; on the other hand certain organotin compounds ( https://en.wikipedia.org/wiki/Organotin_chemistry#Toxicity} are almost as toxic as cyanide.[25]
—————————————-
Extract from;
IBISWorld UK. UK Industry Market Research: Data & Analysis on 400 Industries.
www.ibisworld.co.uk.
What Kind of Cans Are Used for Canned Foods?
by Bonnie Singleton, Demand Media.
The majority of cans used for canned foods around the world today are made from steel. Although there is a thin layer of tin on the surface, there is only 5 to 6 pounds of tin per one ton of steel used.
Canned Controversies.
Almost all metal food and beverage cans contain an industrial chemical called bisphenol A, or BPA. The chemical is used in epoxy resins that coat the inside of food cans. There is a growing concern from scientists and health organizations like MayoClinic.com that BPA can seep into food or beverages. This is a problem because BPA has been linked to prostate and breast cancer, heart disease and damage to the prostate gland of fetuses, infants and children. To avoid this danger, look for cans labeled BPA-free.
But,
BPA vs. BPS Options
Cans need to be lined with something, and you’ll typically have no way of knowing what that “something” is. Often, it’s a similar chemical known as bisphenol-S (BPS).
Unfortunately, BPS is not a safe alternative. Research has shown BPS has estrogenic activity comparable to estradiol, the most potent human estrogen. It was also found to be capable of enhancing estradiol-mediated cell signaling, making it a particularly potent endocrine disruptor.6
Furthermore, the study showed BPS can induce apoptosis (cell death) and interfere with cellular secretion of prolactin (PRL)—a hormone that regulates hundreds of biological functions, including metabolism, reproduction, and lactation.
Originally, BPS was heralded as a suitable alternative because it appeared to be less prone to leaching than BPA… but it must be getting into food because the majority of Americans already have detectable levels in their bodies. Scientific American noted:7
“BPS was a favored replacement because it was thought to be more resistant to leaching. If people consumed less of the chemical, the idea went, it would not cause any or only minimal harm. Yet BPS is getting out. Nearly 81 percent of Americans have detectable levels of BPS in their urine. And once it enters the body it can affect cells in ways that parallel BPA.”
Unfortunately, even if a can (or plastic product) is labeled BPA-free, you can’t be sure it’s also free of BPS. And even if it doesn’t contain BPS, there’s a good chance it contains other harmful chemicals, like phthalates.
Banning BPS will not solve this problem, as there are many types of bisphenols, and simply switching from one to another is nothing but a game of toxic musical chairs.
At present, you may be paying more for a “BPA-free” product that is no safer than the old BPA-containing variety… You’re also exposed to a number of other chemicals courtesy of food and beverage containers, most of which have no warning labels at all.
————-
Fluoridation of water supplies.
More information on this at; http://articles.mercola.com/sites/articles/archive/2015/06/20/fluoride-deception-continues.aspx
This is something that I was aware of at the time that the government decided to add fluoride to public water supplies. Working in the chemical industry it was common knowledge that it was very toxic and was difficult to dispose of. Luckily, our local water company refused to add fluoride to its water supplies. However I have since avoided any products containing fluoride, and advised others to do the same. Obviously I cannot argue that fluoride has effected my health.
Stannous Oxide to replace Fluoride in toothpaste?
Extract from “Tin Based anti-Tumour Drugs”, Edited by Marcel Gielen.
Springer Science & Business Media, 29 Jun 2013. (Page 148+)
A traditional belief that the tin workers in Beauve never suffered from furunculosis led to the use of tin and its compounds in the treatment of staphyloccol infections. In 1917 Frouin and Greigore published a study which suggested that tin, tin oxide, stannous chloride and sodium stannate modified the virulence of staphylocci.
[A traditional belief? Or actual knowledge? A furuncula is a carbuncle and carbuncles are not caused by staphyloccol infection. Brian]
This work, based on seven infected rabbits and two control animals, has since been discredited and more recent evidence indicates that neither soluble nor insoluble tin compound of tin have any effect on staphyloccci in vitro or in vivo.
[I personally discovered this in 1956. The work by Fruouin & Greigore I would assume related to transferring infections from a sufferer of a Furuncula (carbuncle) to the host rabbits. If later tests used infection from a boil or stock staphylocci then obviously no effect would be found. Brian]
Nevertheless, the belief that tin had some value in the control of cutaneous sepsis persisted well into the 20th century and resulted in an occurrence of organotin poisoning in France 1954.
[The belief was that tin was valuable in curing carbuncles/furuncles, NOT cutaneous sepsis. This belief was NOT the cause of the organotin poisoning, which was caused by manufacturing negligence. Brian]
The proprietary compound involved, “Stalinon”, had been contaminated with triethyltin impurities and this resulted in the deaths of more than 100 people.
[This is a different type of belief. This belief was by the medical profession, Cutaneous Sepsis does not apply to carbuncles because the sepsis generally starts in the muscles or fat and it can take many days, even weeks for any signs to appear in the cutaneous layer (Skin) Brian.]
In the UK a preparation containing methyl stannic iodide, “Staniform”, was available before 1958 as an external treatment for staphyloccal infections and an oral treatment containing tin powder and tin(II) oxide “Stannoxyl” was available until recently.
[‘Stannoxyl’ was the last treatment I managed to obtain and was old stock in 1984. At the time I was being treated with antibiotics for weeks with absolutely no effect. A local chemist managed to find a single course of Stannoxyl from wholesalers and within a few days I was cured. Brian]
In contrast to the organotins, metallic tin and inorganic compounds are generally non-toxic with the notable exception of stannane, Sn H4, which is more toxic than arsine.
For many years, stannous compounds have been used in dentistry and oral hygiene. As long ago as 1947, it was known that tin(II) fluoride protected dental enamel from dissolution in lactic acid and since then SnF2 has been incorporated into dentifrices, mouthwashes, topical solutions and occasionally. dental cements.
[In 1945 US started adding fluoride to public water supplies. ]
The compound appears to exert its prophylactic and therapeutic effects in several ways. In combination with acidulated phosphofluoride it allows control of dental caries; it inhibits dental plaque growth; it effectively controls root hypersensitivity; it reduces root surface solubility and finally, it causes less mottling than sodium fluoride.
It has been said that the clinical effect of antimicrobial agents as plaque growth suppressors is not well correlated with in vitro antimicrobial assays of the same agents.
Reports indicate that while both stannous chloride and stannous fluoride possess bacteriostatic effects on oral micro-organisms in-vitro [Laboratory tests outside the body], growth inhibition by SnCl2 in-vivo [actual tests in the body] is only slight. However, no explanation has been offered for these different effects and the results may prove to be anomalies caused by the experimental difficulty in keeping stannous ions in solution.
It has been shown that commercial toothpastes containing SnF2 alone or in combination with Tin(II) pyrophosphate (Sn2P2O7), function as efficient plaque inhibitors and it appears that the Tin(II) [Stannous Oxide] ion is the bacteriostatic agent, with little, if any, effect from the fluoride component. Most of the tin retained in the mouth is bound to the epithelial surfaces, the dental plaque and salivary macromolecules and is slowly released over a period of about 4 hours following brushing, thus allowing a long lasting bacteriostatic effect.
[Therefore there is no excuse for using fluoride in water supplies, toothpaste or mouthwashes.]
Stannous oxide (Tin(II)) is the safest of all the metallic oxides. Aluminium, Copper, Iron, Cromium, manganese, Zinc etcetera can all become toxic outside of certain narrow limits, whereas Stannous Oxide can be overdosed by a large amount with the only symptoms being similar to eating too many apples.
Brian.
———————————-
Essential Minerals
The fact that Tin is not deemed to be an essential mineral is, I feel, due to poor experimental practices. I will explain some of these later.
My main purpose at present is to solve my problem of severe tin shortage in my diet. Normal dietary input is limited to 1-3mg per day against a recommended figure of 10-20mg proposed by Acu-Cell. (Note: Acu-Cell don’t seem to want to sell you any supplements and give no advise on how to get them. I’ve tried)
Most of this is not retained in the body. Adding to this problem are ‘antagonists’, chemicals that reduce the effectiveness of the tin that is retained in the body.
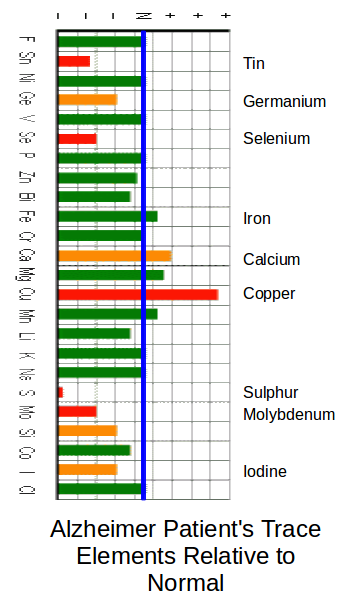
In the Chart below (From Acu-Cell) the blue line indicates the ‘Normal’ for healthy people. Note; The Normal line does not mean that the same amounts (mg) are involved. ‘Normal’ for one element may be 10mg but normal for another element may be 1000mg.
Note; You cannot just increase one item and hope for a cure. A careful balancing is required between multiple elements. You need to go to the Acu-Cell website for much further information.
Brian
In this chart three of the main Tin antagonists are at above normal levels: These are Iron, Calcium and Copper.
Low levels of Tin and Iodine would indicate that there would be some heart efficiency problems.
Low levels of Selenium and Germanium would indicate some brain and nerve problems.
Low levels of Selenium and Sulphur can indicate a wide range of problems including Arthritis and nerve problems.
Note; The extremely low sulphur levels in Alzheimer sufferers tends to highlight a set of problems for the elderly. Egg Yoke is the main natural source of sulphur. Egg Albumin (The White of Egg.) apparently causes constipation in the elderly. Recent medical opinion advises people to eat less eggs. Carers tend not to give eggs to the elderly due to the constipation problem.
—————————————–
Phthalates What are they and What are their dangers?
© 2015 Guardian News and Media Limited or its affiliated companies. All rights reserved.
Phthalates are everywhere, and the health risks are worrying. How bad are they really?
Phthalates are everywhere, and a tidal wave of new research has documented their wide-ranging negative health impacts, but what are the real risks?
Lately, it seems like a new study on the health impacts of phthalates comes out every week. The chemicals are everywhere: they’re used in everything from household cleaners to food packaging to fragrance, cosmetics, and personal-care products.
In 2003, researchers at the US Center for Disease Control documented widespread exposure to a high level of a group of chemicals called phthalates (pdf) across the general American public. The chemicals act as binding agents and also make plastics flexible.
The CDC recommended that the chemicals and their effect on human health be studied further, a recommendation that helped unlock funding for dozens of studies focused on phthalates, resulting in a tidal wave of recently published reports that largely indicate the CDC’s concern was warranted.
The CDC’s warning on phthalates also caught the attention of senators Barbara Boxer and former US representative Henry Waxman, who included the class of chemicals in their Consumer Product Safety bill, passed in 2008. That bill banned the use of some phthalates in children’s products, passed an interim ban on others, and required that the Consumer Product Safety Commission take a close look at the chemicals.
While phthalates is a huge class of chemicals, several have been shown to have negative health impacts.
The resulting report on phthalates – the Chronic Hazard Advisory Panel (Chap) on Phthalates (pdf) – was finalized in late 2014, and despite the chemical industry’s efforts to soften the commission’s recommendations, public health advocates are largely pleased with the effort, a rarity when it comes to government-penned reports on chemical safety.
With academic studies and policy reports consistently voicing concern over the health impacts of phthalates, and consumers beginning to sit up and take notice, regulation may not be far behind.
“The Chap report is the first major regulatory document in the federal government that’s highlighting the extent of the new science on the risks of phthalates,” says Erik Olson, senior strategic director of food and agriculture and health programs for the Natural Resources Defense Council. “The fact that the commission is looking both at phthalates as a group and at the toxicology of individual phthalates is really important,” he says.
Olson was the deputy staff director for the US Senate’s environment and public works committee when the Consumer Product Safety Bill was written and passed. Between the Chap report, a National Academy of Sciences report looking at phthalates as a class and what he calls “the tidal wave of research that’s been coming out fast and furious” in the past year or so, he said, “we’re getting past the phase of complete denial from the industry – they can no longer claim that there’s no risk at all with phthalates.”
What’s the harm?
Name a major public health concern over the past two decades and there’s likely some link to phthalates exposure.
In the past few years, researchers have linked phthalates to asthma, attention-deficit hyperactivity disorder, breast cancer, obesity and type II diabetes, low IQ, neurodevelopmental issues, behavioral issues, autism spectrum disorders, altered reproductive development and male fertility issues.
While phthalates is a huge class of chemicals and nowhere near every chemical in the class has been studied, several have been shown to have negative health impacts: butyl benzyl phthalate (BBzP), dibutyl phthalate (DnBP), di-2-ethylhexyl phthalate (DEHP), diethyl phthalate (DEP), di-butyl phthalate (DBP), benzyl butyl phthalate (BBP), diisobutyl phthalate (DiBP), diisononyl phthalate (DiNP), di-n-octyl phthalate (DnOP), dipentyl phthalate (DPP), di-isobutyl phthalate (DiBP), di-isononyl phthalate (DiNP), di-n-octyl phthalate (DnOP), di-isohexyl phthalate, dicyclohexyl phthalate (DcHP), and di-isoheptyl phthalate.
Enough distinct phthalates have been studied to indicate that companies should proceed with caution when using any chemical in the phthalate class, particularly in products for pregnant women or young children, whom the research has indicated are the most vulnerable to the effects of phthalates.
One of the first phthalates to raise a red flag, DEHP, was replaced in hundreds of consumer products with DiNP, only for researchers to discover a few years later that exposure to DiNP is correlated to male genital birth defects and impaired reproductive function in adult males.
Public health advocates hope to learn from the mistakes made in regulating bisphenol A (BPA) as momentum gathers behind the regulation of phthalates, and ensure that one harmful phthalate isn’t just replaced with another over and over again.
BPA was singled out as the sole chemical of concern in the bisphenol group, and regulated as such. Manufacturers largely replaced BPA with bisphenol S (BPS), which researchers are now discovering is equally as problematic as BPA.
With phthalates, the research has come before any sort of regulation – companies are not even required to list phthalates on consumer product labels – and legislators are already looking at the entire class of chemicals, as well as any particularly bad ones.
No escape
Both because of their ubiquitous usage and because they are not listed on product labels, phthalates are next to impossible to avoid. They are in household items (vinyl flooring), personal care products (hair care, body wash, some cosmetics), fragrance, household cleaners, and food. Even for those who either avoid these products or buy phthalate-free variations, phthalates lurk in unexpected places.
In food, for example, even milk packaged in glass may have passed through plastic tubes on its way from the cow to the bottle, taking DEHP along with it. “Milking machines use a lot of plastic and DEHP is free and very lipophilic (fat soluble), and milk is full of lipids, so it just pulls the DEHP out of the plastic tubing and into the milk,” explains Robin Whyatt, professor of environmental health sciences at the Columbia University Medical Center and the lead author on several landmark phthalate studies. “So my guess would be that milk is a pretty important source of dietary exposure to DEHP.”
Spices are another surprising source of phthalate exposure. A 2013 study, published in the journal Nature, compared the phthalate levels of two groups, one eating their regular diet but armed with a handout of recommendations for ways to reduce BPA and phthalate exposure in their diet, and the other eating a catered diet consisting solely of local, organic fare, none of which had touched plastic packaging. The study authors were shocked to find that DEHP levels in the local, organic group jumped 2,377% over the course of the experiment. Determined to figure out why, the researchers tested all of the foods consumed by the group and found high levels of the phthalate in dairy products and various organic, imported spices.
“The fact is you can’t know if a food has phthalates in it – you can suspect, but it’s almost impossible to know,” Olson says. “That makes them hard to avoid, which is why you need a regulatory framework.”
What now?
Regulation of consumer products moves slowly in the US, and that has proven to be especially true when it comes to chemicals. Despite the recent movement on phthalates, Olson says it is likely to be a long time before we have the sort of wide-reaching framework that would adequately protect the public from harmful exposure.
That doesn’t mean all is lost in the meantime. State and federal regulations have already eliminated the chemicals from some products, and that list is likely to grow. California’s Proposition 65 now includes four phthalates – DINP, DEHP, DBP and BBP – under its labeling requirements, and the state’s Office of Environmental Health Hazard Assessment (OEHHA) recently proposed changes to Prop 65’s warning requirements, which would require manufacturers to list specific chemicals in their warnings and make those warnings more detailed (currently the warnings are vague, stating only “this product [or building] contains substances known by the state of California to cause cancer”).
“Prop 65 will be a driving force for change on phthalates,” Olson says. “Companies don’t like to put warning labels on their products.”
Consumers can also take matters into their own hands by avoiding products packaged in “recycling-code-3” plastic, products that include the vague ingredient “fragrance” on their label, and purchasing organic products packaged in glass as much as possible.
Whyatt also recommends that consumers remove any food packaged in plastic from its packaging and place them in glass. “DEHP continues to leech over time, so you do actually reduce exposure by changing the storage container, even if it’s been in plastic before you bought it,” she says. “All the DEHP has probably not come out yet by the time you get it home. And if there’s still DEHP in there, it’s probably still leeching out, so you can at least reduce your exposure some extent.”
“If we start by addressing the products where we know there’s significant exposure to phthalates, and we start with the most vulnerable communities – pregnant women and children – we can make a real difference,” Olson said. “We could take care of a lot of food exposure through FDA regulation and toys through the Consumer Product Safety Commission, and that’s a lot. It’s not all, but it’s a good chunk.”
Retailers could also play a significant role, as they have with other chemicals of concern. Target and Walmart both launched initiatives to reduce or eliminate toxic chemicals from their shelves last year. Both retailers have said they will make evidence-based purchasing decisions to protect their customers’ health. With a mountain of scientific evidence piling up on phthalates, it can’t be long before consumers begin to put pressure on retailers and retailers in turn push their suppliers to find both alternatives to phthalates and ways to remove the chemicals from their products altogether.
Phthalates can fairly simply be removed altogether from products, with no replacement, according to “green” chemist Bruce Akers. It’s when the chemicals are used to create tubing or packaging that eliminating them becomes tougher: “If you want soft, squeezable plastic, you’re using phthalates,” Akers says.
But according to Whyatt, companies could be using flexible polymers instead. “There are flexible polymers that don’t require a plasticizer – they exist,” she says. “They haven’t been studied really, so we need to know more, but they probably do not leech the way phthalates do. The problem with phthalates as plasticizers is that they’re free floating, they don’t attach to the polymer, so they leech easily. If you have a flexible polymer that shouldn’t happen.”
Despite the size of the issue, Olson remains positive. “We’ve turned a corner on the regulation of phthalates,” he says. “They’re extremely widely used in the economy and it won’t be overnight that we’ll see widespread phase-outs, but clearly we’ve crossed the river and we’re now at the point of debating exactly which uses need to go and where we can use alternatives.”
Correction: This article was updated on 11 February to say Henry Waxman is a former US representative and not a current senator.
© 2015 Guardian News and Media Limited or its affiliated companies. All rights reserved.
More to follow.
———————————–
This is ongoing research in an attempt to save my own life. It may already be too late, but stannous oxide would stop the carbuncle problem within a few days, [See Boil or Carbuncle.] and allow other aspects of my health to improve.
Author; Brian Williams
- At present (4th October 2015). Over the last two weeks I have been taking approximately 200 milligrams each day of tin powder. (Note this is now correct, there was a fault on my electronic weighing unit)
-
External Injuries and Disappearing Bruises.
Posted on March 19th, 2015 No comments“The bruise on the knee is caused by strong gripping.” – Senior Consultant paediatrician.
This refers to a single bruise about 8-10mm dia, on the side of the child’s knee. This bruise was referred to on numerous medical reports. All the medical experts apparently agreed that it was caused by strong gripping.
Note: This bruise was the only one found on the child, apart from severe bruising around the head caused by the birth process. (This head bruising was photographed by the parents shortly after birth).
Later this same consultant referred to this bruise as “bruising around the knee caused by gripping”.
How does ‘strong gripping’ produce a single small bruise?
Statement of parents referring to this bruise; “our dog, a Staffordshire bull terrier, jumped up on to the settee and her paw caught the babies leg.”
Interpretations of this statement in reports given by various medical “experts” on the prosecution team.
A. “the dog jumping up on him and kicked him”. Consultant paediatrician who brought the charges.
B. “the dog jumped up, … her legs were out-stretched and one of them was touching the baby’s knee”. Senior Consultant paediatrician.
C. ” In my opinion the bruise on the knee is unlikely to be caused by the dog resting her feet on the baby in the manner described.” As above.
D. “and it is hard to see how a medium sized dog could land on a child’s knee with sufficient force to cause a bruise”. As above.
E. “the bruise on the knee is caused by tight gripping around the knee”. As above.
The Senior Consultant paediatrician referred to above was from a Children’s Hospital and in affect took over the prosecution.
Dogs have none-retractable claws. We had hoped that this particular consultant would allow us to walk the Staffordshire bull terrier across her naked legs during the court case.
The bruise was too small to have been caused by tight gripping by either of the parents, who were both over 6 feet tall. The bruise was consistent with an impact from a dogs claw.
Although this particular bruise was a genuine bruise that would be recognised as such by most people, various other ‘bruises’ were referred to by the prosecution. These arrived in the months waiting for the court case.
Disappearing Bruises.
Marks that are not bruises.
Petechial ” bruising”.
A very loose definition covering many different situations. If you rest your arm on a ribbed mat for a few minutes, you will find red lines on your arm. According to SBS “experts” this is caused by someone viciously shaking you or throwing you down onto a hard floor. An exaggeration? Yes, but only slightly.
These marks rapidly disappear because no damage has been caused, therefore they are not bruises. This type of marking is caused by pressure, not impact. In fact these marks are not actually ‘petechia’, nor do they have a “petechial appearance” as claimed by most of the paediatric prosecution ‘experts’.
Vasomotor lability
Vasomotor. Causing or relating to the constriction or dilatation of blood vessels.
Lability. Open to change; readily changeable or unstable.
Extract from Consultant Community Paediatrician’s report.
During my assessment, when I lay ******* on the rubber ridged mat to assess his locomotor development, I did notice when I picked him up that he had some red lines on his back which were the impressions from the mat. These disappeared within 5 minutes of finishing the examination.
I felt that this demonstrated a certain amount of vasomotor lability at the skin, but these were clearly not bruises which are caused by trauma and a bleeding into the skin.
This examination was carried out approximately 8.5 months after the child’s birth.
———————————————–
Petechiae Causes.
Tiny blood vessels (capillaries) link the smallest parts of your arteries to the smallest parts of your veins. Petechiae appear when capillaries bleed, leaking blood into the skin. A number of things — including prolonged straining, certain medical conditions, specific types of injuries and some medications — can cause this bleeding.
—————————————————-
The parents took the baby to the family doctor because of a persistent sickness over a few days.
After the examination the doctor noted that red marks were on the baby’s back caused by lying on a ribbed examination table. This was shortly before the ambulance arrived at the surgery to take the baby and parents to the hospital.
At the hospital the baby was examined by two paediatricians. After this the parents were charged with shaking him.
Later reports supplied to the parents ., noted “Dr.********, my colleague, also noted some petechial bruising on the left upper arm…….”.
Why would he say this? He examined the baby quite soon after “his colleague”, so why did he not say that he himself had found these bruises?
There were no petechial bruises on the babies arms at the family doctors surgery, the parents and the baby were with the ambulance staff from the surgery to the hospital. So who caused these “life threatening” injuries due to throwing the baby about?
No subsequent examination found any evidence of this bruising, no member of the family saw this bruising and no photographs were taken of it. Petechial bruising does not just disappear, because it depends on damage to sub-cutaneous blood capillaries.
There “bruises” were marks left by normally handling of the baby, and rapidly disappeared. (Note: The baby was 9 weeks premature at birth). Neither of these two doctors mentioned these arm “bruises” after this report, but they were frequently referred to in other doctors reports and considered to be evidence.
It is a well documented medical fact that due to the lack of development of the blood vessels, the premature infant is at an increased risk of haemorrhage.
External Injuries.
The above mentioned bruise on the baby’s leg was the only external injury found at any time after the baby left hospital after the birth.
However, at birth there was a “very badly bruised head”, as noted by the midwife.
Not a ‘bruised head’, or a ‘badly bruised head’ but a ‘very badly bruised head’.
There was also a depression on the front right hand side of the head.
Author – Brian Williams.
-
Strange statements Made By Medical Experts.
Posted on March 6th, 2015 No commentsA. “The bruise on the knee is caused by strong gripping.” – Senior Consultant paediatrician.
This refers to a single bruise about 8-10mm dia, on the side of the child’s knee. This bruise was referred to on numerous medical reports. All the medical experts apparently agreed that it was caused by strong gripping.
Note: This bruise was the only one found on the child, apart from severe bruising around the head caused by the birth process. (This head bruising was photographed by the parents shortly after birth).
Later this same consultant referred to this bruise as “bruising around the knee caused by gripping”.
How does ‘strong gripping’ produce a single small bruise?
Statement of parents referring to this bruise; “our dog, a Staffordshire bull terrier, jumped up on to the settee and her paw caught the babies leg.”
Interpretations of this statement in reports given by various medical “experts” on the prosecution team.
A. “the dog jumping up on him and kicked him”. Consultant paediatrician who brought the charges.
B. “the dog jumped up, … her legs were out-stretched and one of them was touching the baby’s knee”. Senior Consultant paediatrician.
C. ” In my opinion the bruise on the knee is unlikely to be caused by the dog resting her feet on the baby in the manner described.” As above.
D. “and it is hard to see how a medium sized dog could land on a child’s knee with sufficient force to cause a bruise”. As above.
E. “the bruise on the knee is caused by tight gripping around the knee”. As above.
The Senior Consultant paediatrician referred to above was from a Children’s Hospital and in affect took over the prosecution.
Dogs have none-retractable claws. We had hoped that this particular consultant would allow us to walk the Staffordshire bull terrier across her naked legs during the court case.
The bruise was too small to have been caused by tight gripping by either of the parents, who were both over 6 feet tall. The bruise was consistent with an impact from a dogs claw.
Although this particular bruise was a genuine bruise that would be recognised as such by most people, various other ‘bruises’ were referred to by the prosecution. These arrived in the months waiting for the court case.
Disappearing Bruises.
Marks that are not bruises.
Petechial ” bruising”.
A very loose definition covering many different situations. If you rest your arm on a ribbed mat for a few minutes, you will find red lines on your arm. According to SBS “experts” this is caused by someone viciously shaking you or throwing you down onto a hard floor. An exaggeration? Yes, but only slightly.
These marks rapidly disappear because no damage has been caused, therefore they are not bruises. This type of petechia is caused by pressure, not impact.
———————————————–
Petechiae Causes.
Tiny blood vessels (capillaries) link the smallest parts of your arteries to the smallest parts of your veins. Petechiae appear when capillaries bleed, leaking blood into the skin. A number of things — including prolonged straining, certain medical conditions, specific types of injuries and some medications — can cause this bleeding.
—————————————————-
The parents took the baby to the family doctor because of a persistent sickness over a few days.
After the examination the doctor noted that red marks were on the baby’s back caused by lying on a ribbed examination table. This was shortly before the ambulance arrived at the surgery to take the baby and parents to the hospital.
At the hospital the baby was examined by two paediatricians. After this the parents were charged with shaking him.
Later reports supplied to the parents ., noted “Dr.********, my colleague, also noted some petechial bruising on the left upper arm…….”.
Why would he say this? He examined the baby quite soon after “his colleague”, so why did he not say that he himself had found these bruises?
There were no petechial bruises on the babies arms at the family doctors surgery, the parents and the baby were with the ambulance staff from the surgery to the hospital. So who caused these “life threatening” injuries due to throwing the baby about?
No subsequent examination found any evidence of this bruising, no member of the family saw this bruising and no photographs were taken of it. Petechial bruising does not just disappear, because it depends on damage to sub-cutaneous blood capillaries.
There “bruises” were marks left by normally handling of the baby, and rapidly disappeared. (Note: The baby was 9 weeks premature at birth). Neither of these two doctors mentioned these arm “bruises” after this report, but they were frequently referred to in other doctors reports and considered to be evidence.
It is a well documented medical fact that due to the lack of development of the blood vessels, the premature infant is at an increased risk of haemorrhage.
————————————–
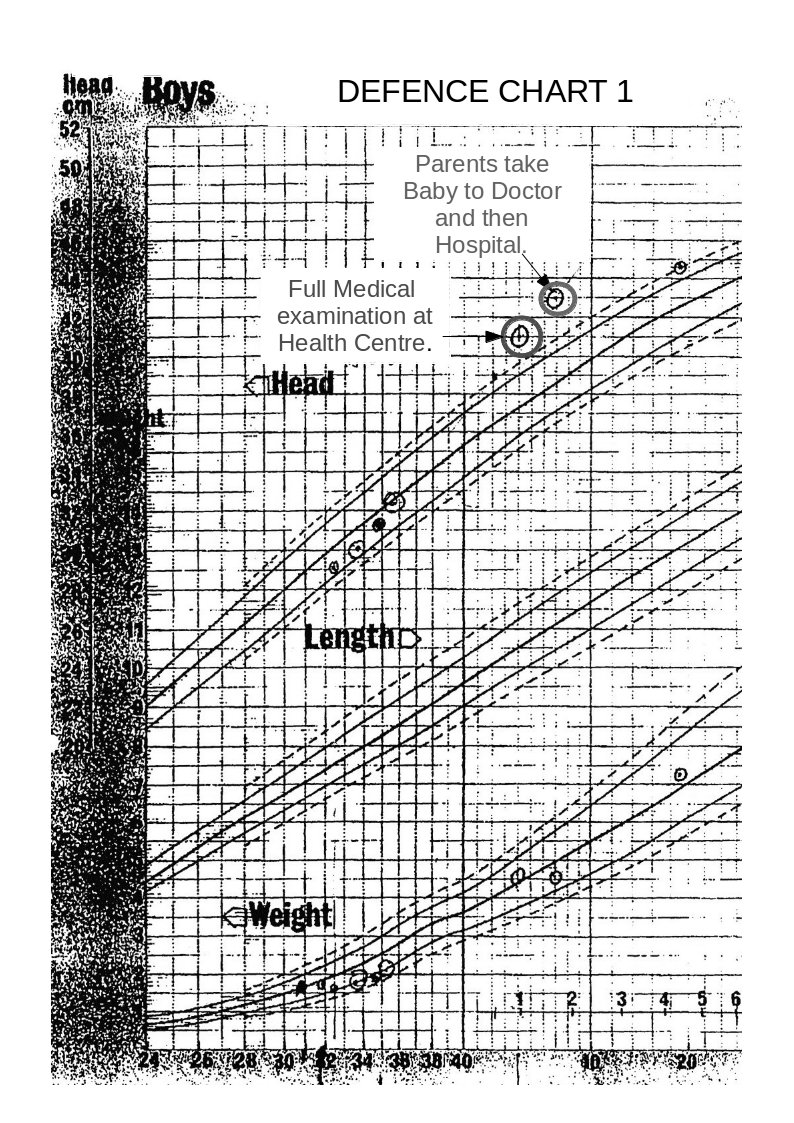
B. “Measurements of the baby’s head circumference during its stay in the Special Care Baby Unit at *******, demonstrate that *****’s head circumference increased at a normal rate during the first few weeks of life, and that **** head size was in proportion to body weight. ” – Senior Consultant paediatrician.
Why do I consider this to be a strange statement to make?
Because the baby’s head circumference was increasing at a rate twice that of a normal birth baby, 4 days before it was released from hospital into the parent’s care. Also, the weight was falling seriously relative to the guide curves (centile charts).
Note; The above graph shows ‘Centile’ curves which are standard guide curves, used to monitor child growth. The centre line on each set of curves is the 50th centile (the ideal curve), the lower dashed line is the 10th centile and the solid line above this is the 25th centile. The upper dashed line is the 90th centile and the solid line below this is the 75th centile.
On the weight curves the measurements show that the child’s weight is actually falling relative to the 50th centile, and is in the danger area. (Whoever entered these point measurements obviously got them wrong at first and then corrected them.)
On the head measurement curves the figures indicate that the head diameter is rising.
In fact the head measurements between the last two made whilst the baby was still in hospital shows that the rise in the period was twice that of a normal ‘term’ baby.
Any doctor should have seen that this was a serious cause for concern.
Was this Senior Consultant Paediatrician lying?
Had this Senior Consultant Paediatrician not seen the charts?
I personally believe that the charts had not been seen.
Note on the above graph the two high-lighted measurements. The measurement taken at the heath centre, 3 weeks before the parents took the child to the doctor and hospital, shows that the head measurement was well above the 97th centile danger level at this time.
This timing is important and will be discussed later.
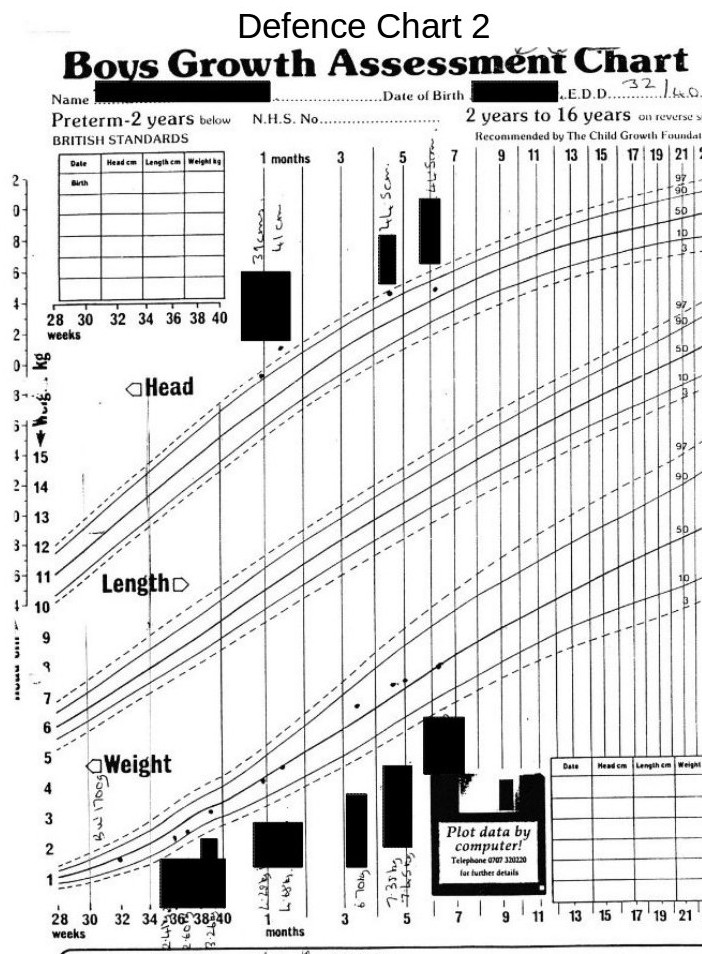
The defence team received a copy of this chart 5 months after the parents had been charged, despite constant requests for medical records.
The graph below shows a centile graph received at the same time as the previous chart, but obviously created at a later date. You will notice that there are differences.
The head circumferences figures are not now shown for the period from birth to leaving hospital. Also, the plotted point on Defence Chart 1 at the Medical Centre that was shown to be well above the danger level has now been moved down to the 90th centile line.
Strange!
Medical Incompetence or Medical Fraud?
The following chart is on produced by someone on the prosecution team and issued to at least some of the medical/surgical experts in the prosecution team. The family received this attached to the back of a copy of a report issued by one of the medical experts.
Again we see differences between this and the original charts. The difference between the low weights shown on the original charts and weights shown on this chart, which seem to have mysteriously moved towards the 50th centile, are obviously suspicious.
The head circumference figures (whilst still in hospital), are now lost in a vague area without, any clear definition but indicating that they are on a straight line roughly on the 50th centile.
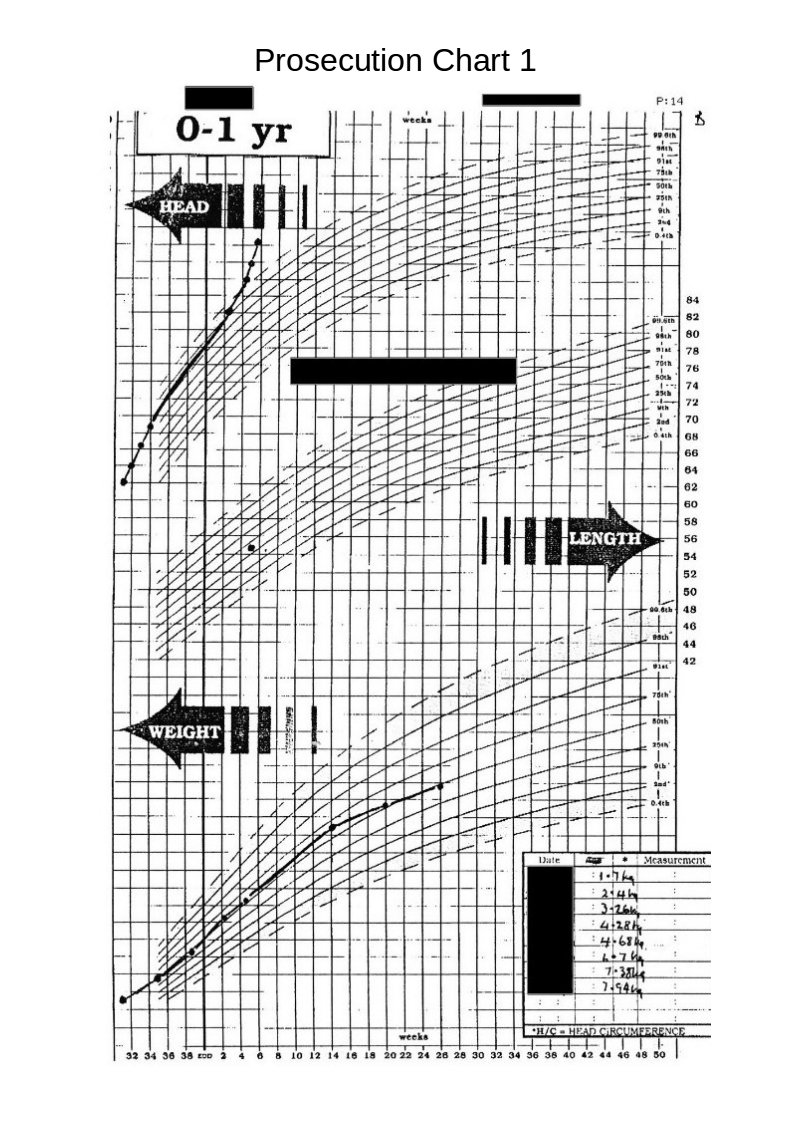
The following chart, (received with the above chart ) caused me much astonishment and gave me a good, much needed, laugh.
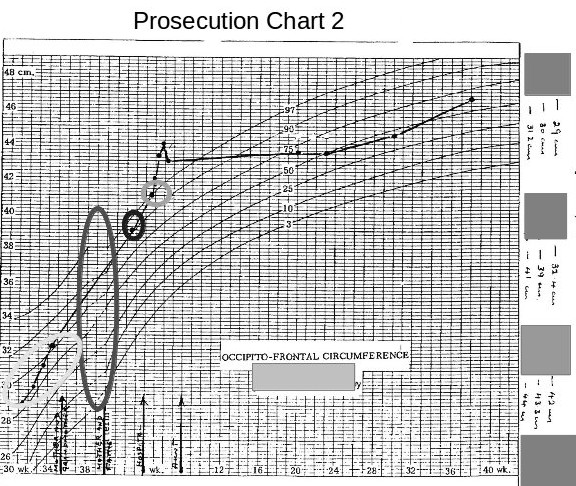 The most obvious thing at first glance was that the chart was a fake made up from two different charts, the long ellipse highlighting the join between the two graphs.
The most obvious thing at first glance was that the chart was a fake made up from two different charts, the long ellipse highlighting the join between the two graphs.The second most obvious error is that this chart now shows all the head measurements, taken whilst the baby was in hospital, have now magically moved to above the 50th centile. (I would imagine that this is a medical impossibility for a baby being born 9 weeks premature and having a birth weight of 1.7 kilogrammes.)
The third interesting item is that the measurement taken at the health centre and the one taken later at the hospital, have now fallen considerably.
These discrepancies mean whoever produced these charts was grossly incompetent or that these charts produced by the prosecution are deliberate fraud.
Now compare the above chart with the one below.
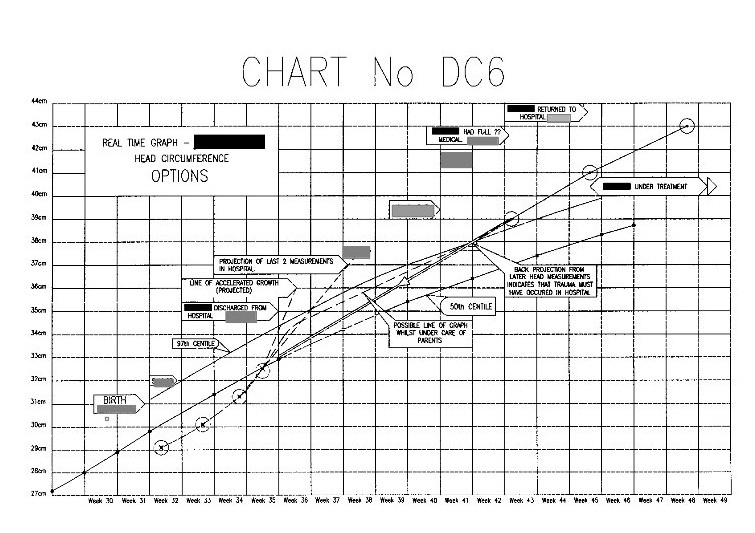 This is one of the explanatory charts I produced for the defence report.
This is one of the explanatory charts I produced for the defence report.All of the actual seven head measurements taken in this period are in the correct positions relative to the time scale. The horizontal divisions are in weeks, and the vertical divisions are in 1cm intervals.
Obviously it looks totally different from the previous prosecution one.
Let us look closer at this chart.
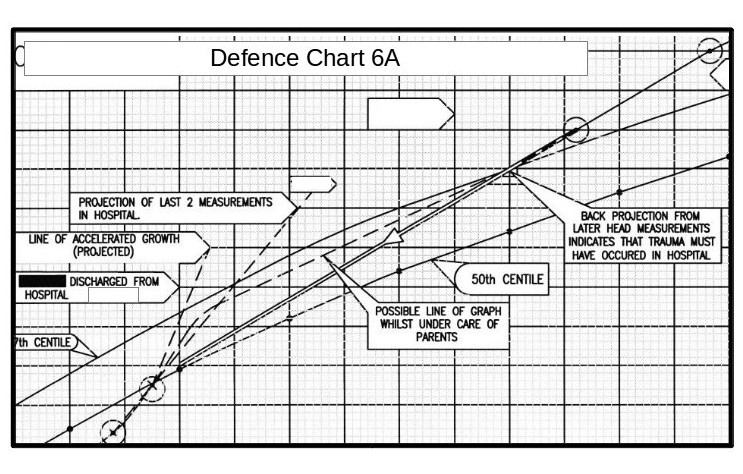 The measurement in the top right hand corner is that taken after the parents took the baby to the doctor and then hospital. The next measurement down is that taken at the Medical Centre two weeks before. All the paediatricians stated that some incident must have happened between these dates (i.e throwing the child about or throwing him onto a hard surface) to account for for the head swelling.
The measurement in the top right hand corner is that taken after the parents took the baby to the doctor and then hospital. The next measurement down is that taken at the Medical Centre two weeks before. All the paediatricians stated that some incident must have happened between these dates (i.e throwing the child about or throwing him onto a hard surface) to account for for the head swelling.However, projecting a straight line between these these two points backwards, brings it almost exactly to the last measurement made in the hospital.
Author – Brian Williams.
-
Nasty Tricks by the SBS Prosecution Team
Posted on January 24th, 2015 No commentsShortly before the start of the Court Hearing the Social Services Department (SS) asked the accused parents to attend a meeting with them, to apparently discuss what would happen during the hearing. They were told that they did not need members of their families or legal representation to be present. (NOTE; Prior to this meeting members of the families and/or a legal representative were always present, a condition insisted on by the parents and family.)
It should also be noted that the family had, from May******* onwards, persisted in requesting that the evidence gathered by the family should be read and considered. The request was consistently refused by the SS., stating that all the evidence could only be presented and considered at the trial. Even the “Expert” consultant appointed by the court to represent the parents refused to look at the evidence gathered by the family.
The only none family members with detailed knowledge of this evidence were the family solicitor, (provided by the court), The Guardian-ad-Litem (provided by the Council and representing the child) and myself.
Leading up to this stage, the family had stated on various occasions that they would bring counter-charges against the social services on the grounds of incompetence and failing to carry out their duty to investigate. (This is documented)
During this latest meeting the parents were told that the court hearing would be cancelled if the parents would sign a document absolving the Social Services from any faults. It should be noted that parent’s solicitor and the family were unaware of this meeting until much later.
At this time both the parents were severely stressed and in no state to consider this sensibly and signed the document.
What caused this sudden magnanimity by the Social Services?
As the social services had consistently refused to discuss the evidence, I feel that the Guardian-ad-Litem must have warned them that to take the case to court would cause them serious repercussions.
The Court hearing was cancelled.
Was this the happy ending for the family?
No.
More on this later.
Author – Brian Williams
-
The Full Mathematics of the Michelson – Morley Experiment. Part 1
Posted on November 28th, 2014 No commentsI have never understood why the physics establishment decided that time differences were relevant to the Michelson – Morley experiment. I first looked at this about 48 years ago and my first thoughts were that there could not be any time differences. I am now absolutely certain. The actual results obtained from the experiment are exactly in accordance with the laws and mathematics of classical mechanics.
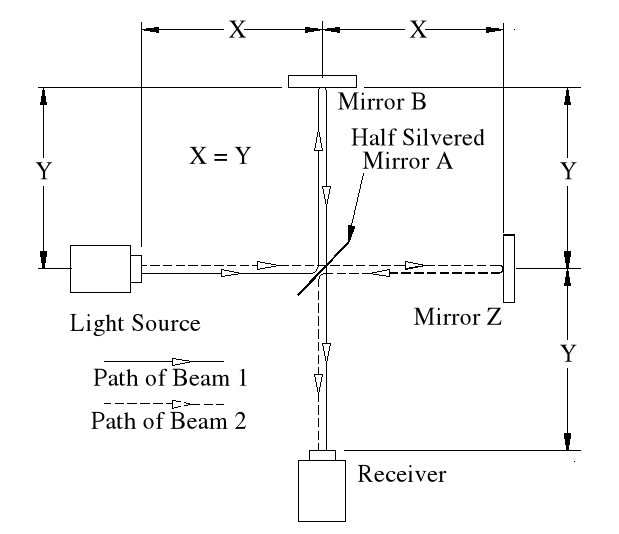
Basic Set-up of the Experiment.
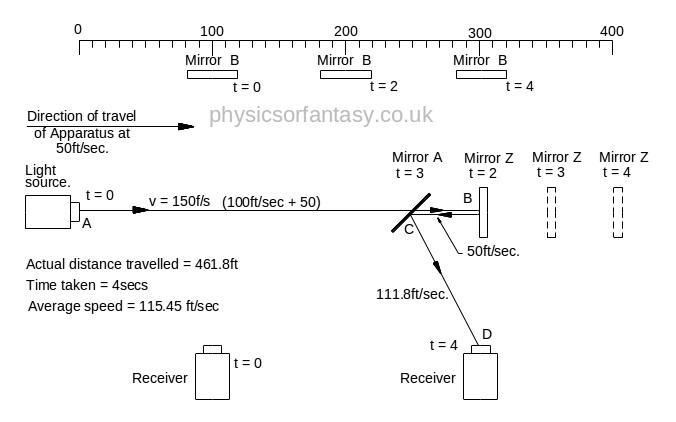
The idea behind this experiment, dreamed up by Michelson & Morley, was that somehow it would enable them to detect the motion of the Earth in space, (or relative to a fixed point in space), and that it could be detected by differences in the time taken for two beams, travelling different paths, both identical in length. No differences were observed. Note that the physicists assumed that the path lengths were identical. This was the first major error of the Michelson-Morley experiment.
Unfortunately, the experiment (in 1897) was doomed to failure, because the mechanics of it were not understood by Michelson & Morley, (or any later physicists.)
Summary of Actual Mathematics and Mechanics.
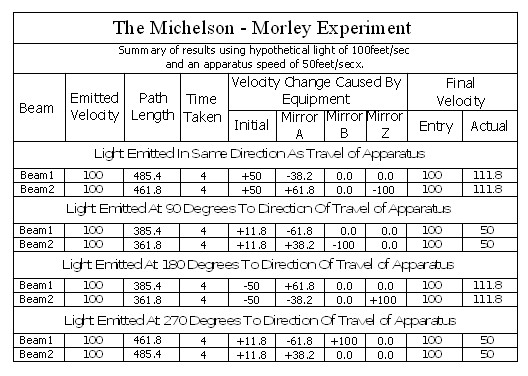
Note; This is simply an experiment in mechanics, and therefore all the laws of mechanics must be strictly adhered to.
In the above chart ‘Emitted Velocity’ means the velocity that the light travels away from the light source.
Let us consider that the apparatus is travelling in the same direction as the light is emitted. Let us assume that the speed of light is 100feet/sec, and let us assume that the apparatus is travelling at 50feet/sec. I do this because using the actual figures leaves us with messy figures such as;
” T2 = (2 x 0.1 x 300,000.0015/300,000) x (1/300,000.0015)” – From Physics or Fantasy – Section 1. (It would also be difficult to get figures like this into the chart above.)
Beam 1
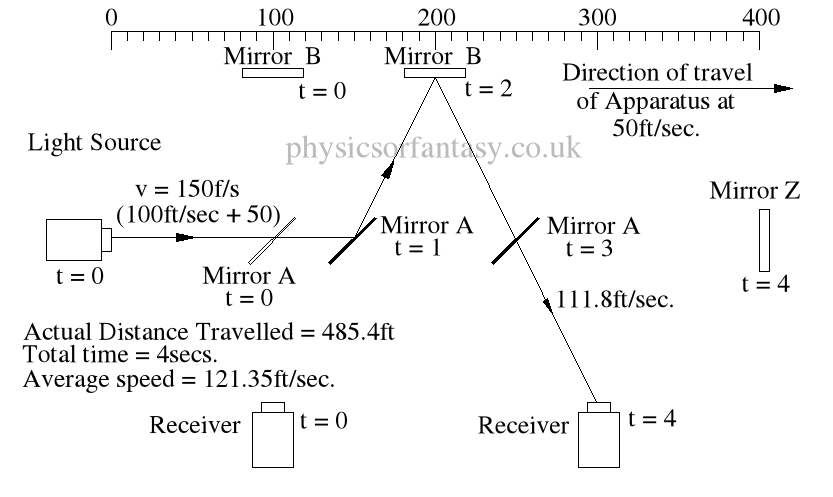
Within the apparatus the light would travel a distance of 400 feet in 4seconds, travelling from the emitter to Mirror A, then on to Mirror B and then passing through Mirror A again until reaching the Receiver.
However, whilst this is happening, the apparatus will have travelled 200 feet up the laboratory at a velocity of 50feet/second in the same 4 seconds.
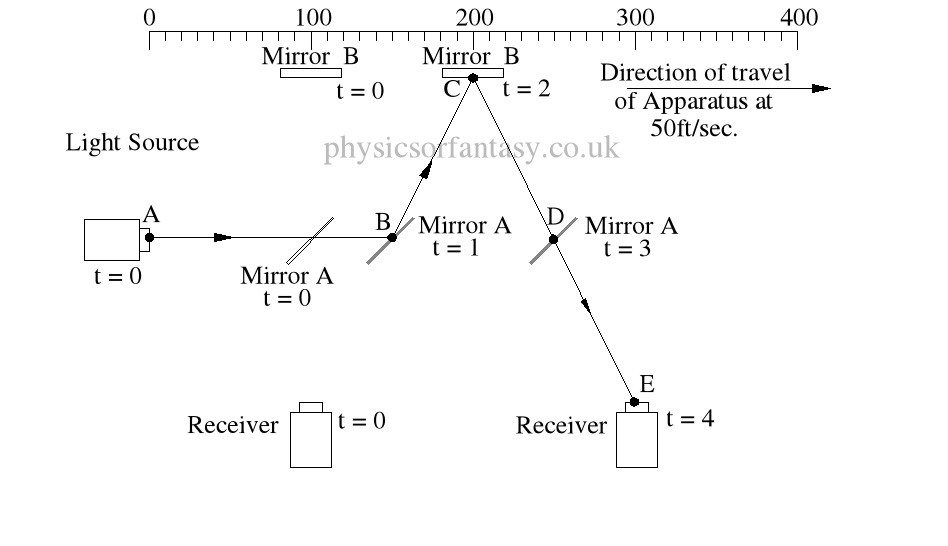 Ignoring the speed of the light and considering only the speed of the apparatus, the light must pass through the points B, C, D and E, after being emitted from point A, and at the times stated. The distance the light must travel to pass through these points is 485.4 feet. If the apparatus is moving at any speed relative to any point outside the apparatus, then the distance travelled relative to this external point must be greater or less than the path length within the apparatus, in our example 400 feet.
Ignoring the speed of the light and considering only the speed of the apparatus, the light must pass through the points B, C, D and E, after being emitted from point A, and at the times stated. The distance the light must travel to pass through these points is 485.4 feet. If the apparatus is moving at any speed relative to any point outside the apparatus, then the distance travelled relative to this external point must be greater or less than the path length within the apparatus, in our example 400 feet.The light is emitted at time t = 0, and must arrive at point E because that is where the receiver will be at time t = 4secs.
The velocity of the light relative to its starting position (Or even a fixed point in space) will be 100 ft/sec emission velocity plus 50ft/sec (the velocity of the apparatus), but only until it strikes Mirror A.
Because Mirror A is moving away from the light, and is at 45° to direction of travel, the reflection of the light is, (according to the laws and mathematics of classical mechanics.) reduced to a velocity of 111.8 ft/sec. In other words the actual velocity of the light is reduced by 38.2 ft/sec.
Anyone who plays tennis, cricket, table tennis etc. will understand why this happens, without needing a degree in physics. A batsman facing a 100mph ball has various options. He can play a forward forcing shot (which will increase the speed of the ball) over the bowlers head, hopefully, for six runs. He can also move the bat backwards at the time of impact, which will reduce the speed of the ball. In both situations the angle of the bat also affects both the speed and direction of the ball.
Mathematics
Va = Velocity of Apparatus.
Ve = Emitted Velocity of the light.
mA = Mirror A, mB = Mirror B, mZ = Mirror Z.
PD = Physical distance between items of apparatus, example: PD(E>mA) = physical distance between emitter and Mirror A. This is 100ft in all cases in these examples.
TD = Travel distance of light between leaving one item and arriving at the next.
E = Light emitter.
R = Receiver.
Note: It is irrelevant whether you use feet, metres, miles, kilometres or Mega-miles as your units of measurement, if you remember to be consistent. This post is designed to investigate the validity of Einstein’s mathematics and logic.
Beam 1 – (E>mA)
This is the simplest part of the mathematics.
If the light is emitted at 100ft/sec. and the apparatus is travelling at 50ft/sec then the light must travel at 150ft/sec.
In 1sec. Mirror A will have travelled 50ft from a position 100ft in front of the starting position of the light.
In other words, in 1sec. Mirror A will be 150ft away from the starting position of the light.
If the light travelled a a constant speed of 100ft/sec, it would take 1.5 secs to arrive at the position that Mirror A was after 1 sec. However, it would still be 25 feet behind Mirror A.
Time taken from Mirror A to Mirror B.
Tan A = Actual distance moved by Mirror B, divided by the physical distance between Mirror A and Mirror B.
Tangent A = 50/100 = 0.5. Angle A is therefore 26.565°
TD = Cosine 26.565° = 100/X = 111.8 ft.
It must travel this distance in 1 sec. otherwise it it would miss mirror B. ( Note; Although the mirrors in my diagrams are shown as very wide, in actual practice the mirrors would be narrow, probably about 0.5 inches (12.5mm) wide).
The actual distance travelled by the light from light source to Mirror B is now 150 + 111.8 = 261.8ft. It has taken 2 seconds.
The light now has to travel from mirror B to the receiver R. The actual distance between mirror B and the receiver R is 200ft. However, the light has to travel a distance of 2 x 111.8 to arrive at receiver R.
It takes 2 seconds to travel this distance at a speed of 111.8ft/sec.
TD(E>R) = TD(E>mA) + TD(mA>mB) + TD(mB>E) = 150 + 111.8 + (2 x 111.8)
= 485.4ft
The average speed of the light is 485.4/4 = 121.35ft/sec.
It should be obvious that over a period of 4 seconds the light must strike mirror A then Mirror B, then Mirror A again, and finally the receiver R, at the times shown because that is where the items are at the times shown. The light beam must travel at the speeds shown to allow it strike the items.
Beam 2.
Beam 2 travels from the emitter E to Mirror Z, then back to Mirror B and then onto the Receiver R.
The path length (measured on the equipment) is 400ft.
Note: The light strikes mirror Z at a closing velocity of 100ft/sec, because mirror Z is moving away at 50ft/sec. The light reflection loses another 50ft/sec. because the mirror is still moving away at 50ft/sec.
The speed of the light reflection relative to mirror Z is 100ft/sec. Confusing isn’t it? But it is true. Cricketers, table tennis players, etcetera all use this fact whilst playing their games, and without a honours degree in physics or engineering. Although not an everyday problem in mechanics it does arise quite often in machinery design.
TD(E>mZ) = Time taken from Emitter E to Mirror Z.
= TD(E>mZ)/V2(E>mZ) = 300/150 = 2 sec.
DT(mZ>mA) = Time taken from Mirror Z to Mirror A.
= TD(mZ>mA)/V(mZ>mA) = 50/50 = 1 sec.
T(mZ>R) = Time taken from Mirror Z to Mirror A.
= T(mZ>R)/V(mZ>R) = 111.8/111.8 = 1 sec.
—————————————————
Notes on lack of time differences in the Michelson – Morley experiment.
If you place the experiment on a train and rotate it through 360° and measure the time taken for the light to travel from the emitter to the the receiver at various angular positions, no time differences will be detected.
——————————————–
If you are in a car travelling eastwards towards the rising Sun at a road speed of 100 mph, then your speed relative to the Sun (It being a ‘fixed point in the universe relative to us), would be approximately 1,100 mph. (The earth rotates at approximately 1000 mph.)
If later the same day on the same road and still travelling at 100 mph, but travelling in a westerly direction towards the setting sun, your speed relative to the sun would be approximately minus 900 mph. Therefore your speed relative to the sun would have changed by 2000 mph, (i.e. from +1100mph to -900mph.) in the 12 hours between the two journeys.
——————————-
If you replace the car with a beam of light and carry out similar timings the light will actually be travelling at c + 1000 mph when travelling towards the sunrise, and c-1000mph when travelling towards the sunset, ( c = The claimed constant speed of light.) The velocity of the light (Relative to the Sun) changes by 2000mph between these
Let us change our apparatus to a tube 300,000 kilometres long (approximately). Inside this tube there is a light transmitter(A) at one end and a light receiver(B) at the other end. We set this tube travelling towards the sun, with end B at the leading end, at a speed of 300,000 kps. When B is at a distance of 300,000 kilometres from the sun we fire the light emmitter at A, towards B (the receiver). One second later end B hits the sun at exactly the same time as the light hits (B). At the time (A) is triggered it is 600,000 kilometres away from the sun.
During this one second the light has travelled 600,000 kilometres and this light has travelled at twice the speed of light!
If we had reversed the tube so that A was at the front end and carried out the same procedure, the light would travel from A to B in two seconds. (end B is still 300,000 kilometres from the sun when end A hits the sun). The light still travels the length of the tube in one second but to an outside observer the light front would be static whilst the end B would be travelling at 300,000 kps towards the light front.
It is this aspect of the Michelson – Morley Experiment that confuses the physicists (plus one or two trigonometry mistakes.)
The man plus the train, the car plus the road, are just different versions of the Michelson – Morley experiment.
Author – Brian Williams
-
Goethe’s Colour Spectra.
Posted on November 8th, 2014 No commentsYesterday, 7th November 2014 I was searching Google Images for suitable photographic images to add into my post “Williams’ Pin Prism, and Fraunhofer Lines.”. This was not an easy task because most images do not show the true visual evidence and are more computer graphics than reality. However, the task was definitely worthwhile, because I came across an image originally produced by Johann Wolfgang von Goethe. (1749 – 1832)
Most modern images are similar to the ones below.
These images are totally against the reality of what happens with prism spectra, and I find it very annoying , especially when being used to teach people.
Note; I have been sent this video clip from a reader who asked me to explain it.
http://www.youtube.com/watch?v=7Fl0GZsBhGo&NR=1
I am afraid that the contents of it demonstrate the poor teaching and lack of honesty in modern physics.
The drawings below, originally created by Goethe. and re-created by www.handprint.com are very close to the actual/true situation. His method/style used is identical to that used by myself, so he definitely beat me to it.
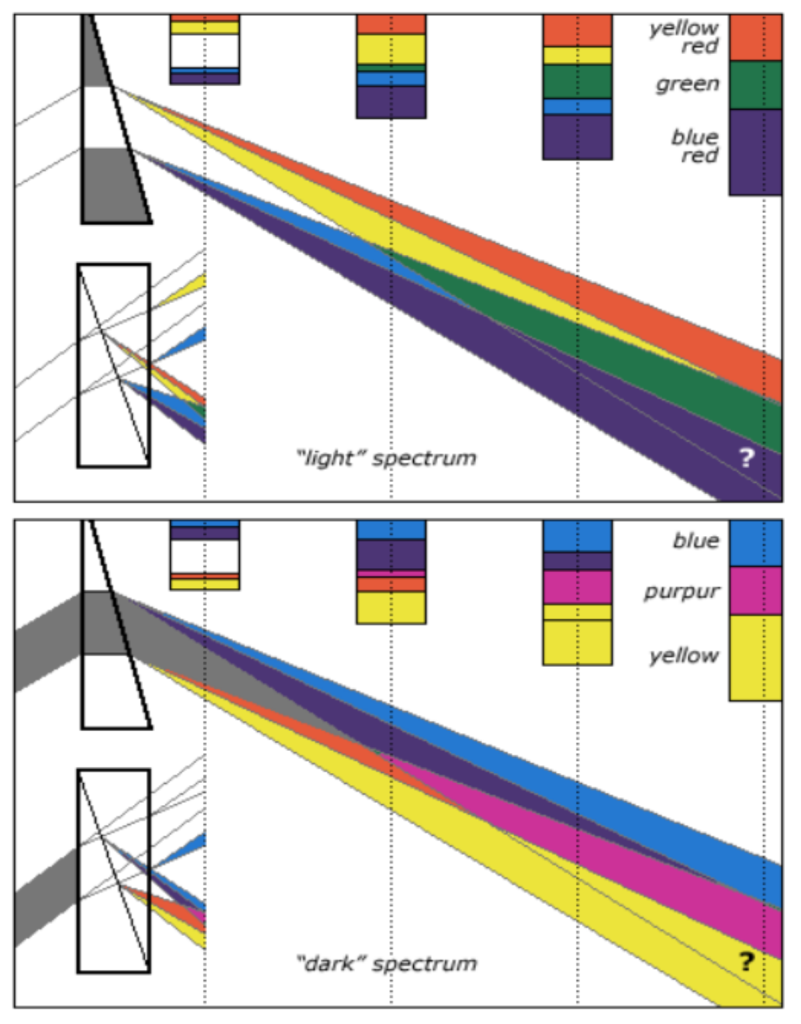 handprint : goethe’s “zur farbenlehre” www.handprint.com380 × 555Search by image
handprint : goethe’s “zur farbenlehre” www.handprint.com380 × 555Search by image“light” and “dark” spectral mixtures from. Goethe’s “primordial” fringes adapted from Plate IV, Figures 1 and 2 and ¶214-¶216 of Zur Farbenlehre (1810)
I assume that the above colours are based on the actual colours seen in the original book, which will have faded. In the top picture the Green shown should be a very bright Green.
The lighter Blue should be Cyan.
Where the question mark is, this area should be a dark Green.
The top picture (“Light” spectrum) is what I refer to as an “Expansion Spectrum”.
The bottom picture (Dark” spectrum) is what I refer to as a “Compression Spectrum”.
In this picture, where the question mark is, this area should actually be very pale Mauve or White. “Purpur” should actually be Magenta. Magenta only occurs in compression spectra and Greens only occur in expansion spectra.
Note; It is the slit, (that constricts the light into a beam), that actually creates the colours. The White beam entering the prism has thin Yellow + Red + Blue edges to it. This is why the colours start at edge of the spectra exiting the prism, as shown in the above drawings. What is missing from the top drawing is the dark Blue band. Its energy is measurable and is named “Infra Red” due to lack of knowledge, by the physicists. It is missed because this ‘true’ spectra Blue is very dark and is ‘lost’ against the dark background. The lighter Blues are a mixture of either Blue and White or Blue and Red.
In the drawing below, the left hand side (White) is the source of the light. This light passes through an aperture (Slit) and shines onto the prism.
 In passing through aperture, narrow coloured edges are created on each side of the beam of light. These edges are composed of three colour bands, Blue on the outer edge, Red in the middle and Yellow on the inside These edges are created by the Laws of Fluid Mechanics. If you look under “Orifices” in your text book on Fluid Mechanics, all should be explained.
In passing through aperture, narrow coloured edges are created on each side of the beam of light. These edges are composed of three colour bands, Blue on the outer edge, Red in the middle and Yellow on the inside These edges are created by the Laws of Fluid Mechanics. If you look under “Orifices” in your text book on Fluid Mechanics, all should be explained.See also my “Taper Slit Experiment” and my “Taper Silhouette Experiment.
Author – Brian Williams
-
Williams’ Pin Prism, and Fraunhofer Lines.
Posted on November 5th, 2014 No commentsI am sorry, but the spectroscope does not indicate any atomic information about a material. As a comparator it is a very useful tool but only in tightly controlled conditions.
It does not tell you the atomic structure of a distant object, nor does it tell you the atomic structure of an object held within the spectroscope equipment itself.
The Pin Prism.
I carried out these experiments in 1990. My idea was to look at the mechanics of the prism. I know that most people will say that a prism is just a triangular piece of glass and that a slit is just a rectangular hole but, individually, they change light. The slit creates the colours and the prism creates the spectrum. Working together, they become a mechanism.
Basically, the Pin Prism is (was, the water rapidly destroyed it) a triangular array of pins. The original one consisted of brass ‘panel pins’ hammered into a plastic coated plywood board. The array quality was rather erratic because hammering pins into this material is very difficult when trying to get them at 3mm centres. There were other problems in getting the experiment working, but I will discuss them later. (Note; I carried out similar experiments with a ‘Pin Lens’ and a ‘Pin Sheet’ that also gave quite good results considering the poor quality of the equipment.) Note; You obviously do not need to spend £millions of public money to carry out physics research.
Most of the results roughly matched the comparison with the light experiments i.e. where the ‘Yellow and Cyan ‘ bands overlapped the water was very turbulent, and the ‘Yellow and Red’ areas plus the ‘Blue’ areas were fairly smooth.
However, I finally noticed that were various gaps in the flow. Initially I was just intrigued and carried on experimenting, varying the flow rates and noting what happened. I noticed that the gaps lengths extended with increase in flow rate.
As the flow rate dropped the gap diminished until it disappeared. As the flow reduced even more, a ‘ridge’ formed, caused by the water that was originally on both sides of the gap combining. Therefore the water flow changed from a gap to a ‘pressure ridge’.
With a fixed experimental set-up with light, if we start off with a high energy light source, sufficient to produce black lines in the spectrum, and slowly reduce the energy , the gaps in the flow (black lines) will reduce in width and length until the gaps (black lines) disappear.
If we continue reducing the energy level, bright lines will develop to replace the dark lines. If the original black line was in an area of the spectrum that was red, then a brighter red line would develop.
What causes the gaps in the spectrum on which so much physics research is based?
The answer is, the same mechanics that cause the gaps in the pin prism. The water flowing through the pin prism follows a very complex path. On entering the prism it is split into a number of streams by the first pins. Whilst passing through the prism it is split or combined on numerous occasions before exiting the prism.
If the angle at which the water enters the pin prism is constant then the paths followed by the individual streams will tend to be constant.
The speed of any particular stream of water will depend on the energy loss due to the length of the path taken.
With light the same mechanics apply. The atoms of glass act like the pins in the pin prism. The various streams of light are split or combined thousands of times before the light exits the prism.
See also “Goethe’s Colour Spectra”
Author – Brian Williams
Abstract from Physics or Fantasy – Section 2 – Colour and The Quantum Theory
-
Liquid Balance on Saturn Satellite ‘Mimas’
Posted on October 17th, 2014 No comments16 October 2014
‘Death Star’ moon may be ‘wonky or watery’
From the BBC News WEB Site.’
“The internal structure of one of Saturn’s moons is either wonky or awash with water, according to a new study. Mimas is nicknamed the Death Star because it resembles the infamous Star Wars space station. It has a tell-tale wobble that is twice as big as expected for a moon with a regular, solid structure. The researchers offer two explanations: either it has a vast ocean beneath its surface, or a rocky core with a weird shape resembling a rugby ball. The study appears in Science Magazine. Its authors are astronomers in the US, France and Belgium, who based their calculations on high-resolution photos of Mimas snapped by the Cassini spacecraft. Cassini was sent to Saturn in 1997 to explore the planet and its many moons, which so far number 62 (53 with names). Exotic interior The researchers built a detailed 3D model of Mimas using images taken from various angles, and tracked the movement of hundreds of reference points on its pockmarked surface. “After carefully eFrom the BBC News WEb Site.’xamining Mimas, we found it librates – that is, it subtly wobbles – around the moon’s polar axis,” said lead author Dr Radwan Tajeddine, who works at Cornell University in the US. Apart from these gentle “librations”, Mimas otherwise presents the same face to Saturn throughout its orbit.”
—————————————–
Liquid Balancing is a recognized method of balancing rotating objects.
Earth,s oceans provide a very successful balancing mechanism for any imbalances due to the Earth’s structure.
On a rotating object , a liquid automatically arranges itself to offset any imbalances.
Even the subsurface magma will try to re-arrange the floating landmasses to offset any imbalances.
I do not think that the scientists can expect to find much water on ‘Mimus’ and I suggest they take plenty of water with them when they go.
Author – Brian Williams.