-
How to Build an Atom – The Mechanics.
Posted on August 27th, 2015 No comments“So far I have been unable to find any property of matter that cannot be explained by the use of only electrons and nuclei.”
———————————————————
The construction of an atomic model that satisfies all the requirements of scientific knowledge regarding both physical and biological facts must be our starting point. It must be able to explain colour, weight (mass), state (i.e. solid, gas or liquid), changes due to temperature, changes due to pressure, hardness, and softness, rigidity and flexibility, chemical reactions, toxicity, radioactivity, gravity, magnetism, and most importantly, life.
My theory explains all known properties of matter. (It therefore passes the requirements of a theory.)
I may be wrong, but certainly not as wrong as the Physics Establishment.
The reader will have problems due to fact that I go against all the current atomic hypotheses that he/she will have been taught or accepted. I can understand this because I had the same problem myself, many times over the years, finding it difficult to believe my own results. Eventually, it was easier to consider existing hypotheses only to pinpoint where the problems were. This was a case of selecting any particular hypothesis proposed by the physics establishment, assume it is wrong, and work out alternative hypotheses and then produce a working theory. 90% of current hypotheses cannot satisfy the title of theory because there are no explanations of how they could work.
One of the main reasons for physicists opting for high-speed electrons is to try to explain the energy of an atom. However, all energy is in one of two types/states, momentum or stress. (Note; In rotary motion the energy is stored in a combination of momentum and stress.) All atoms are in a state of stress. The reader may find this statement difficult to accept but it is true, whether you are considering chemistry, radiation or mechanics.
Fig. 1 below shows the image of the atom best known by the public.
Fig. 2
 Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.
Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.Neither of these models is of any use in explaining the known properties of matter. How do two or more atoms form a molecule without all the electrons colliding? How can one atom be harder than another? How is one atom a liquid and another a solid?
Ask your local physicist any of these questions and he will mumble that it is too complicated to explain to mere mortals. The fact is that modern physics cannot explain any of them.
If electrons are not whizzing (whizzing is travelling at high speed) around causing dangerous collisions then it is easy to form molecules. All you have to do is to remove one electron from one atom and fill the gap by moving another atom so that one of its electrons fits in the gap in the first atom. Dead easy, you now have a molecule, two atoms jointed by a mutual electron. Both atoms have the right number of electrons therefore nothing has changed but we now have a molecule. However, life, physics and mechanics are not that simple.
Modern physics argues that the more electrons there are in an atom then the heavier it is. Therefore, as our molecule is an electron short of two atoms, it should be lighter than the original two atoms. Unfortunately, I totally disagree with this hypothesis because it is illogical.
The Structure of Atoms and the origin of Mass.
If Hydrogen is the lightest element and only has one atom, the one to one bond between the nucleus and the electron would make it far more difficult to remove this one electron from Hydrogen than trying to remove one electron from an atom with many electrons. (See Proton.) However, Hydrogen is the easiest element from which to obtain energy in the form of emitted electrons. In addition, Hydrogen has a ‘soft’ explosion indicating that the velocity of emitted electrons is relatively low. Although there are a few arguments (but not facts) against this, none of them overcomes the other problems of an atom with orbiting electrons.
If we reverse the hypothesis and say that the greater the number of electrons the lighter the atom, then this explains away many of the problems in physics without introducing any more. This would mean that the heaviest possible atom would have one electron.
It would also mean that the lightest detectable element, Hydrogen, would have the second largest number of electrons.
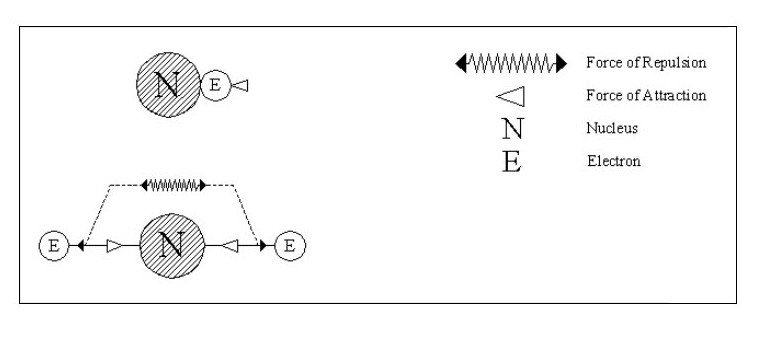
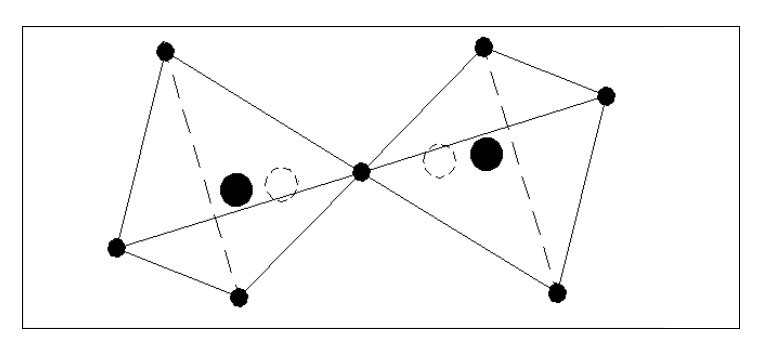
Let us consider some simple possible atoms. In Fig. 3, the top sketch shows a nucleus with a single electron. As this electron is not whizzing around it snuggles up against the nucleus due to the force of attraction. The lower sketch shows an atom with two electrons. In this case, the force of repulsion between the two electrons is acting against the force of attraction between the electrons and the nucleus. The electrons would align themselves on opposite sides of the nucleus. Although I have shown the electrons standing well clear of the nucleus, they could still be pressing against the nucleus but with less force. This would depend on the relative strengths of the attraction and repulsion forces.
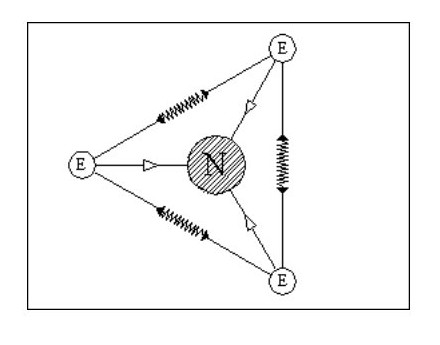
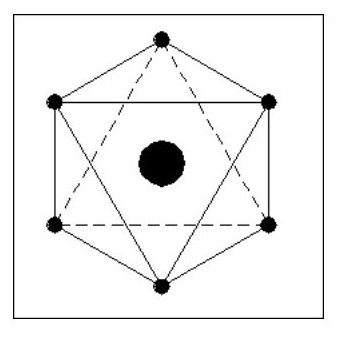
Let us add another electron. In Fig. 4, the three electrons have arranged themselves around the nucleus so that the forces of attraction and repulsion are balanced.
Now let me explain the problem that is irritating you. How can we have flat atoms? If the atom was a mile away from the nearest other atom or available electrons then Fig. 4 would be its natural state. In close proximity to other atoms, it would tend to combine with these atoms to form complex molecules, or try to accumulate more electrons.
It should be remembered that Fig. 4 is not a Lithium atom, it is an atom that is extremely heavy and one, like our previous two atoms, only being found at the core of collapsed stars or far out in space.
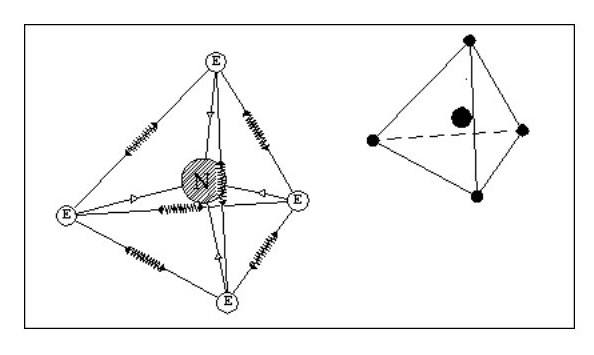
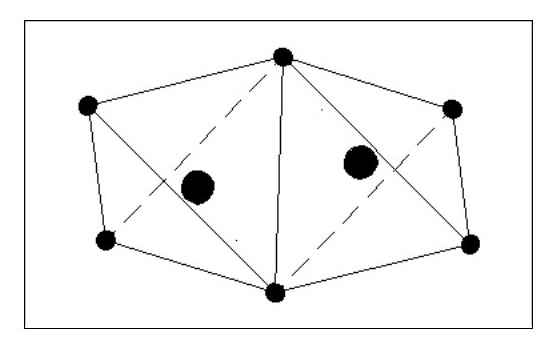
Let us now consider an atom with four electrons. Fig. 5 shows the atom in its ‘natural’ state.
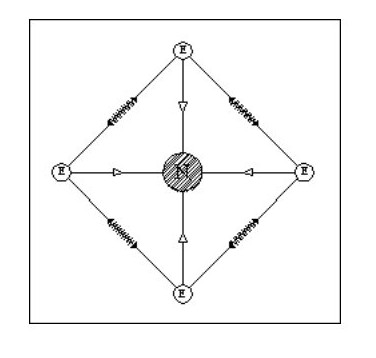 However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6
However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6Now that we have arrived at our first three-dimensional atom let us consider molecules. Using Fig. 6, we can create a simple molecule by using a single common electron.
With this type of molecular bond, it is obvious that the molecule will be quite flexible because one atom can flex and rotate relative to the other.
We now come to another deviation from modern physics. It is considered that for electrons to be attracted to the nucleus, the nucleus must have a positive charge (See Proton). Looking at the evidence from most experimentation would indicate that:
A. The nucleus is neutral and that the electron is attracted to the neutral state. Or
B The nucleus is attractive to both electrons and other nuclei, but the surrounding electrons reduce the affinity for other nuclei.
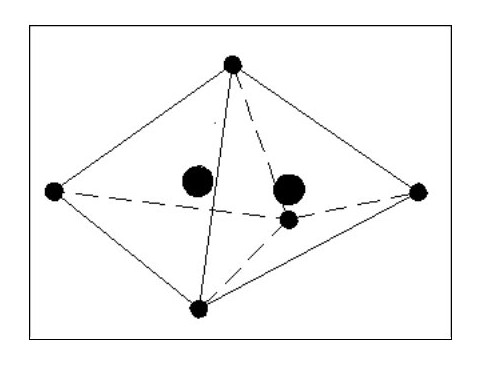
I feel that B. is the most logical. In the above sketch (Fig. 7) the shared electron presents a weakness in the surrounding shield of electrons, and the mutual attraction between the nuclei would pull them closer together as shown by the dotted circles. This in turn would distort the shapes of the two atoms as the forces balance. This weakness would be present in all molecules with similar results.
Let us now consider that these two atoms are joined as a molecule but by two common electrons instead of one. The formation of this molecule releases two electrons, therefore making the molecule heavier than the previous molecule. This new molecule is also less flexible than the previous one, one atom effectively being hinged off the other.
It should be considered that we are still dealing with extremely heavy atoms at this stage and the removal of electrons requires great pressure or a high energy.
If we now take our two atoms and create a molecule using 3 common electrons ( See Fig. 9 Below), we lose most of the flexibility and have an even heavier (and harder) molecule. It is clear that from two identical atoms we can produce the option of 3 different molecules each having different properties.
Now consider this point, if we split this molecule we are 3 electrons short of our original two atoms.
We could end up with one of our original atoms plus an atom with a single electron.
The modern physicist would argue that this result proves that the molecule was created by the addition of two totally different atoms. If it was difficult or impossible to split the molecule then the modern physicist would argue that this proves that the molecule is actually an atom!
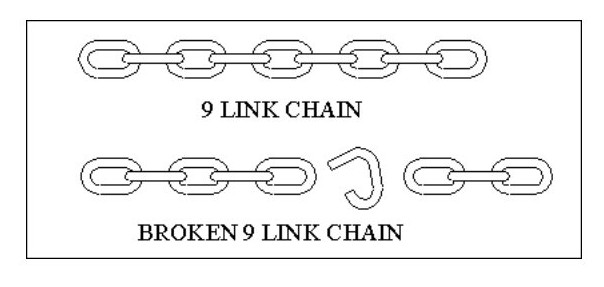
Most of our known substances considered to be atoms may in fact be difficult to split molecules, and many of our molecules may be conglomerations of identical atoms, but which when separated produce two or more totally different atoms. In the sketch below if the chain breaks as shown you could mistakenly argue that the 9 link chain is obviously created from a 5 link chain and a 3 link chain.
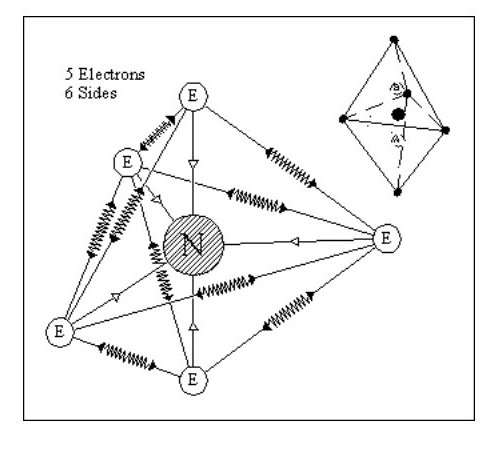
Let us now consider an atom with 5 electrons. We have a problem with this in that it is difficult to balance all the forces. On the other hand it gives us a glimmering of the reason for the life force.
Although similar in shape to Fig. 9 it only has one nucleus which can move between A and B with little effort, seriously distorting the shape of the atom in the process. This distortion would not destroy the atom, the overall forces being strong enough to retain its integrity.
It would take little effort to set up an oscillation in this atom. If an oscillation was set up and the atom shielded from all external forces, the oscillation could continue forever.
See Atomic Damping.
We are still looking at very heavy atoms, the weight/mass detectable being dependent on the number of electrons. The actual mass of an atom remains unchanged as this is the mass of the nucleus, which does not change. The electrons mask the affect of the nucleus’ mass. With a full complement of electrons the atom will have no detectable mass.
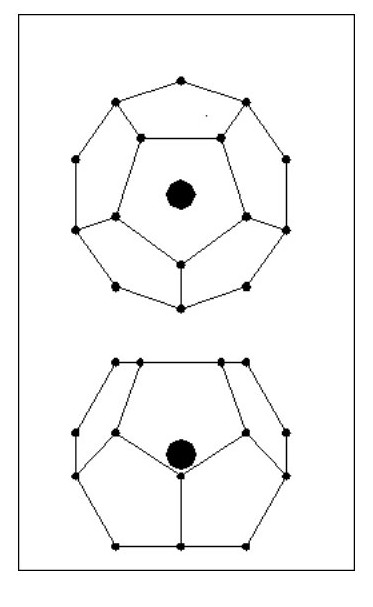
We will now look at a lighter atom. We will consider an atom in the shape of a Dodecahedron i.e. 12 sides and 20 electrons as Fig. 14.
(You might also like to look at The Origin of the Golden Section., because ratios relating to the dodecahedron tie in with golden section ratios.)
All the electrons lie on a sphere, equally spaced and all the same distance from the nucleus. Obviously 20 electrons will not mask the affect of the nucleus’ mass. [Note: With regard to the dodecahedron shape, this would clearly allow for molecular bonding with 1, 2 or 5 common electrons. However, under certain conditions 3 and 4 electron bonds could occur.]
Can we increase the numbers of electrons without causing problems?
If we add 12 electrons they will be attracted to the nucleus but will have problems with the repulsion forces of the existing electrons. These repulsion forces are weakest in the centres of the faces so the new electrons will align themselves in the centre of the faces.
But consider the following point: The existing electron sphere reduces the attraction forces for the new electrons and each new electron has to contend with the repulsion forces of 5 existing electrons. Our original dodecahedron will now have a five sided spike on each face as Fig. 15.
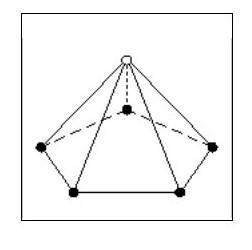 It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.
It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.The new electrons will be equally spaced around the outer shell and will all be the same distance from the nucleus.
Now if we can have 12 electrons in a shell outside the dodecahedron, can we have a shell inside it containing 12 equally spaced electrons? The answer is yes. We can also have another shell on the outside containing 20 electrons, followed by further shells having alternatively 12 and 20 electrons.
The limit will be reached when the number of electrons completely mask the attraction force of the nucleus.
Interim Recap.
The orbiting electron hypothesis does not explain any of the properties of matter. See note.
- My theory explains rigidity and resilience.
- My theory so far gives a faint insight into the possible cause of the life force.
- My theory gives a logical cause for the crystal structure of many materials.
- My theory explains molecular bonding.
- My theory explains weight/mass. See note.
Note: The existing hypothesis states that the number of electrons governs the mass/weight of the atom. It does not explain how. The original reason for the statement that hydrogen had one electron was not based on any scientific reasoning or facts.
My readers may be wondering when I am going to explain about quarks, pi-mesons, etc. etc.
The answer is that there would be no point in wasting valuable space. The majority of these are hypothetical particles dreamed up to try and balance the faults in the existing weak hypotheses. Note; So far I have been unable to discover any property of matter that cannot be explained using only electrons and nuclei.
Let now consider an atom with six electrons. This would be in the form of 8 triangular faces.
As with the dodecahedron we could add an extra shell of electrons, in this case 8 of them. These electrons would would form a slightly distorted cube. If we add further shells they each would have 8 electrons until the forces blanketed the nucleus.
Now to answer your question as to how many electrons does it take to blanket the nucleus. I just don’t know. The lightest known substance is hydrogen. No-one knows how many electrons each atom of hydrogen has despite the physicists statement that it has only one.
The problem is that we really don’t know how many electrons any atom has. None of the experiments in the last 100 years has produced any logical figures and the standard tables of the elements can only be considered as pure guesswork.
We will take an arbitrary figure of 100 as the number of electrons required to blanket the nucleus.
Logically it would only take one additional electron to finally trip an atom into a condition that would make it undetectable. In the above atom the electrons are added in batches of 8. In the dodecahedron shaped atom the electrons are added in batches of 12 or 20. Obviously both families of atoms could get to the stage of being undetectable. It would appear to be unlikely that in either case would the final shell would need a full complement of electrons before the atom became undetectable.
However it just may require a full complement or at least a balanced part complement.
The Structure and Mechanics of Atoms and Mousetrap molecules
Let us consider chemistry and chemical reactions. Chemistry relates to the formation of molecules.
If you put powdered copper, zinc, carbon, titanium plus water into a tray and mix them, once they have settled you will still have powdered copper, zinc, carbon, titanium plus water in the tray. No new molecules will have been created. Why not?
Cu + C + Zn + Ti + H20 = ?????
You cannot get an answer for the above equation because it does not allow for the two most important variables; Temperature and pressure. (I’ve never come across a valid formula for a cup of tea either.)
Without variables no formulae are valid.
Under ‘natural’ conditions certain molecules can be created. Obviously ‘natural’ conditions vary depending on where you are. Natural conditions at the North Pole are different than natural conditions in the Sahara Desert. ‘Natural’ conditions on the Moon are different conditions than on Pluto or Mercury. Under ‘natural’ conditions many molecules cannot be created i.e. they need high /low pressure or high/low temperature or a combination.
Under ‘non-natural’ conditions many molecules can be created.
Most life on Earth evolved to suit gradually changing ‘natural’ conditions. Occasionally, unnatural conditions, such as volcanic activity occur that release molecules that have been created under unnatural conditions such as high temperatures and high pressures. Many of these molecules are toxic to most forms of life on Earth. Some lifeforms, such as the sea creatures around volcanic vents on the ocean floors, have evolved to handle the toxins, high pressure and high temperatures. To these creatures these conditions are now ‘natural’.
Under higher pressures atoms contract and under lower pressures atoms expand. If the pressure is high enough electrons are ejected from atoms (Exothermic reaction). THEREFORE they become different atoms that have qualities different from the original atoms.
Lighter atoms compress more than heavy atoms, therefore they will lose electrons easier than heavy atoms.
The gas Nitrogen has a completely different atom structure than liquid nitrogen.
Carbon and diamond have different atomic structures.
Water and Ice have different atomic structures.
If you place two dissimilar atoms that, (under normal conditions would not form a molecule), under increasing pressure you will arrive at condition such that the lighter atom would be able to form multiple electron bonds (MEBs) with the heavier one.
When this happens the molecule would be stable at this particular pressure. However, if the pressure is released, the molecule will either split into two separate atoms again or will remain as a molecule but with the lighter atom being in a distorted and stressed condition. This would make it vulnerable to forming MEBs with other atoms that it would not be possible under normal conditions.
Mousetrap Molecules
In affect, the molecule could become a ‘mousetrap molecule’ that could be easily triggered into forming strange/unnatural molecules or atoms, or even molecular explosions. Apart from toxins this would explain ‘radio activity’ and inflammability.
Radioactivity depends on a source of materials that have been subject to high pressures. Most of these are relatively stable in that only a few molecules per/second explode in any given mass. However, when they do the lighter atom disintegrates at high speed. Impurities in the ore tend to restrict the amount of radioactivity. Purifying the ore increases the ratio of mousetrap molecules until they start to trigger each other and you get a sustained reaction
————————————–
NOTE; Free Electrons have a very important role in chemistry. Free electrons are those not locked into the structure of an atom. They may be attracted to nearby atoms but can be easily moved around without affecting the structure of the atoms. Free electrons will still have a blanketing/insulating effect on the nucleus of the atom that would hide its true mass.
(Structural Electrons are those electrons that are part of the structure of atoms.)
Heat and Temperature.
Let me make the following statement: ATOMS DO NOT HAVE A TEMPERATURE.
Any isolated atom is completely devoid of any sense of temperature, it is neither hot, warm or cold.
If it loses an electron then this electron MAY be detected as heat.
Under pressure, atoms will reach a stage when the atomic structure starts to break down and electrons are emitted.
Temperature measurement in this case is dependent on its emission velocity.
——————————————————-
The mass of an atom is a force.
Let me apologise for the title of this post. You cannot build an atom. You can only disassemble an atom.
I was going to change the title but could not think of a better one.
———————————————-
Families of Atoms
MORE TO COME SHORTLY
Author; Brian Williams
Comments accepted.
-
Individuals names only, no company names.
-
Only one URL allowed.
-
Note that comments are now processed through a
separate computer.
Comments automatically deleted. (I do not see them.)
-
All comments from pornographic sites.
-
All comments from sales sites.
-
All comments not relevant to my website.
-
Nasty Tricks by the SBS Prosecution Team
Posted on January 24th, 2015 No commentsShortly before the start of the Court Hearing the Social Services Department (SS) asked the accused parents to attend a meeting with them, to apparently discuss what would happen during the hearing. They were told that they did not need members of their families or legal representation to be present. (NOTE; Prior to this meeting members of the families and/or a legal representative were always present, a condition insisted on by the parents and family.)
It should also be noted that the family had, from May******* onwards, persisted in requesting that the evidence gathered by the family should be read and considered. The request was consistently refused by the SS., stating that all the evidence could only be presented and considered at the trial. Even the “Expert” consultant appointed by the court to represent the parents refused to look at the evidence gathered by the family.
The only none family members with detailed knowledge of this evidence were the family solicitor, (provided by the court), The Guardian-ad-Litem (provided by the Council and representing the child) and myself.
Leading up to this stage, the family had stated on various occasions that they would bring counter-charges against the social services on the grounds of incompetence and failing to carry out their duty to investigate. (This is documented)
During this latest meeting the parents were told that the court hearing would be cancelled if the parents would sign a document absolving the Social Services from any faults. It should be noted that parent’s solicitor and the family were unaware of this meeting until much later.
At this time both the parents were severely stressed and in no state to consider this sensibly and signed the document.
What caused this sudden magnanimity by the Social Services?
As the social services had consistently refused to discuss the evidence, I feel that the Guardian-ad-Litem must have warned them that to take the case to court would cause them serious repercussions.
The Court hearing was cancelled.
Was this the happy ending for the family?
No.
More on this later.
Author – Brian Williams
-
The Full Mathematics of the Michelson – Morley Experiment. Part 1
Posted on November 28th, 2014 No commentsI have never understood why the physics establishment decided that time differences were relevant to the Michelson – Morley experiment. I first looked at this about 48 years ago and my first thoughts were that there could not be any time differences. I am now absolutely certain. The actual results obtained from the experiment are exactly in accordance with the laws and mathematics of classical mechanics.
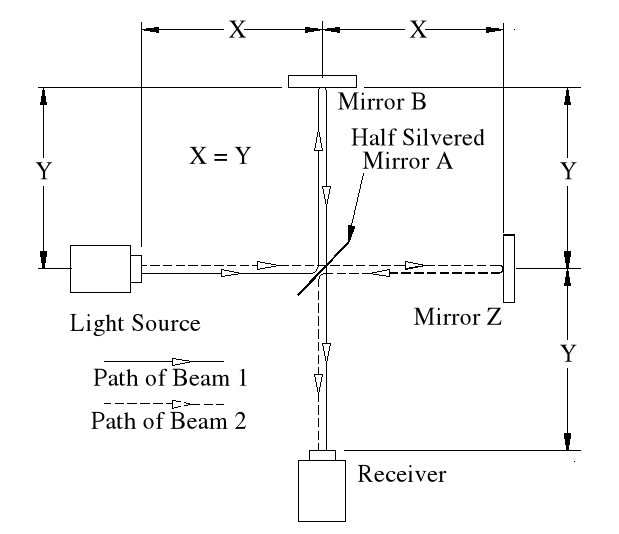
Basic Set-up of the Experiment.
The idea behind this experiment, dreamed up by Michelson & Morley, was that somehow it would enable them to detect the motion of the Earth in space, (or relative to a fixed point in space), and that it could be detected by differences in the time taken for two beams, travelling different paths, both identical in length. No differences were observed. Note that the physicists assumed that the path lengths were identical. This was the first major error of the Michelson-Morley experiment.
Unfortunately, the experiment (in 1897) was doomed to failure, because the mechanics of it were not understood by Michelson & Morley, (or any later physicists.)
Summary of Actual Mathematics and Mechanics.
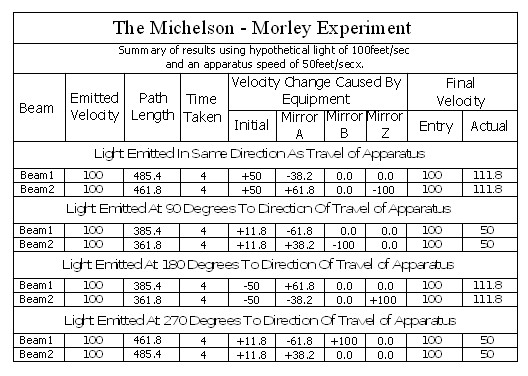
Note; This is simply an experiment in mechanics, and therefore all the laws of mechanics must be strictly adhered to.
In the above chart ‘Emitted Velocity’ means the velocity that the light travels away from the light source.
Let us consider that the apparatus is travelling in the same direction as the light is emitted. Let us assume that the speed of light is 100feet/sec, and let us assume that the apparatus is travelling at 50feet/sec. I do this because using the actual figures leaves us with messy figures such as;
” T2 = (2 x 0.1 x 300,000.0015/300,000) x (1/300,000.0015)” – From Physics or Fantasy – Section 1. (It would also be difficult to get figures like this into the chart above.)
Beam 1
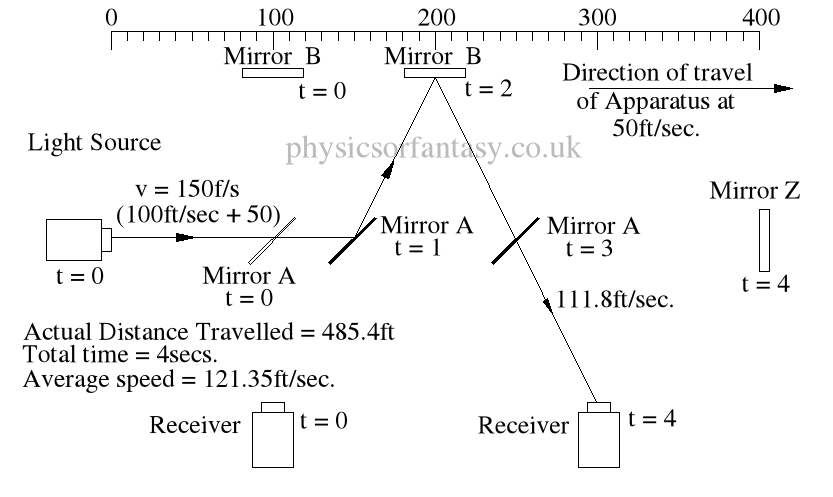
Within the apparatus the light would travel a distance of 400 feet in 4seconds, travelling from the emitter to Mirror A, then on to Mirror B and then passing through Mirror A again until reaching the Receiver.
However, whilst this is happening, the apparatus will have travelled 200 feet up the laboratory at a velocity of 50feet/second in the same 4 seconds.
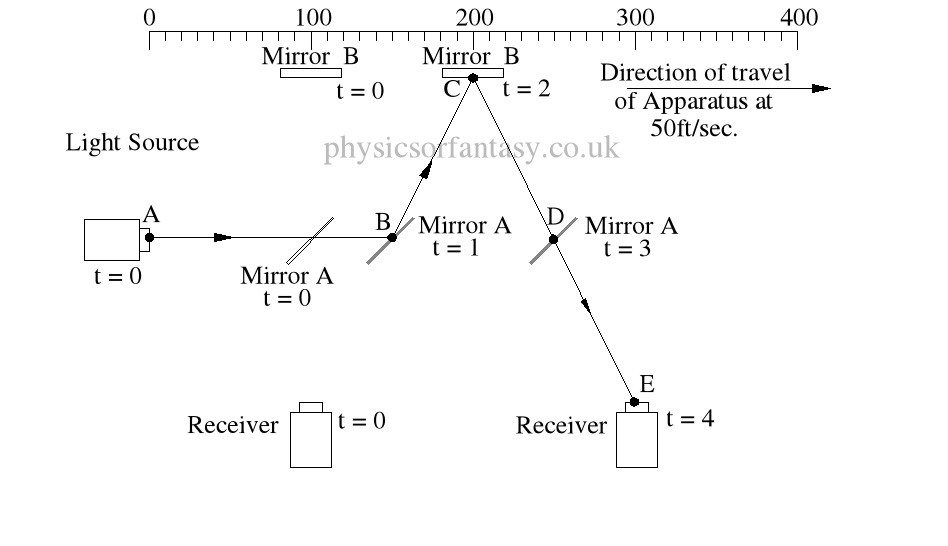 Ignoring the speed of the light and considering only the speed of the apparatus, the light must pass through the points B, C, D and E, after being emitted from point A, and at the times stated. The distance the light must travel to pass through these points is 485.4 feet. If the apparatus is moving at any speed relative to any point outside the apparatus, then the distance travelled relative to this external point must be greater or less than the path length within the apparatus, in our example 400 feet.
Ignoring the speed of the light and considering only the speed of the apparatus, the light must pass through the points B, C, D and E, after being emitted from point A, and at the times stated. The distance the light must travel to pass through these points is 485.4 feet. If the apparatus is moving at any speed relative to any point outside the apparatus, then the distance travelled relative to this external point must be greater or less than the path length within the apparatus, in our example 400 feet.The light is emitted at time t = 0, and must arrive at point E because that is where the receiver will be at time t = 4secs.
The velocity of the light relative to its starting position (Or even a fixed point in space) will be 100 ft/sec emission velocity plus 50ft/sec (the velocity of the apparatus), but only until it strikes Mirror A.
Because Mirror A is moving away from the light, and is at 45° to direction of travel, the reflection of the light is, (according to the laws and mathematics of classical mechanics.) reduced to a velocity of 111.8 ft/sec. In other words the actual velocity of the light is reduced by 38.2 ft/sec.
Anyone who plays tennis, cricket, table tennis etc. will understand why this happens, without needing a degree in physics. A batsman facing a 100mph ball has various options. He can play a forward forcing shot (which will increase the speed of the ball) over the bowlers head, hopefully, for six runs. He can also move the bat backwards at the time of impact, which will reduce the speed of the ball. In both situations the angle of the bat also affects both the speed and direction of the ball.
Mathematics
Va = Velocity of Apparatus.
Ve = Emitted Velocity of the light.
mA = Mirror A, mB = Mirror B, mZ = Mirror Z.
PD = Physical distance between items of apparatus, example: PD(E>mA) = physical distance between emitter and Mirror A. This is 100ft in all cases in these examples.
TD = Travel distance of light between leaving one item and arriving at the next.
E = Light emitter.
R = Receiver.
Note: It is irrelevant whether you use feet, metres, miles, kilometres or Mega-miles as your units of measurement, if you remember to be consistent. This post is designed to investigate the validity of Einstein’s mathematics and logic.
Beam 1 – (E>mA)
This is the simplest part of the mathematics.
If the light is emitted at 100ft/sec. and the apparatus is travelling at 50ft/sec then the light must travel at 150ft/sec.
In 1sec. Mirror A will have travelled 50ft from a position 100ft in front of the starting position of the light.
In other words, in 1sec. Mirror A will be 150ft away from the starting position of the light.
If the light travelled a a constant speed of 100ft/sec, it would take 1.5 secs to arrive at the position that Mirror A was after 1 sec. However, it would still be 25 feet behind Mirror A.
Time taken from Mirror A to Mirror B.
Tan A = Actual distance moved by Mirror B, divided by the physical distance between Mirror A and Mirror B.
Tangent A = 50/100 = 0.5. Angle A is therefore 26.565°
TD = Cosine 26.565° = 100/X = 111.8 ft.
It must travel this distance in 1 sec. otherwise it it would miss mirror B. ( Note; Although the mirrors in my diagrams are shown as very wide, in actual practice the mirrors would be narrow, probably about 0.5 inches (12.5mm) wide).
The actual distance travelled by the light from light source to Mirror B is now 150 + 111.8 = 261.8ft. It has taken 2 seconds.
The light now has to travel from mirror B to the receiver R. The actual distance between mirror B and the receiver R is 200ft. However, the light has to travel a distance of 2 x 111.8 to arrive at receiver R.
It takes 2 seconds to travel this distance at a speed of 111.8ft/sec.
TD(E>R) = TD(E>mA) + TD(mA>mB) + TD(mB>E) = 150 + 111.8 + (2 x 111.8)
= 485.4ft
The average speed of the light is 485.4/4 = 121.35ft/sec.
It should be obvious that over a period of 4 seconds the light must strike mirror A then Mirror B, then Mirror A again, and finally the receiver R, at the times shown because that is where the items are at the times shown. The light beam must travel at the speeds shown to allow it strike the items.
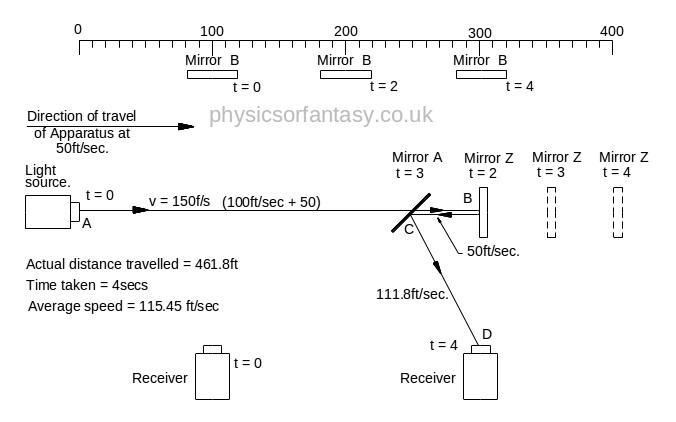
Beam 2.
Beam 2 travels from the emitter E to Mirror Z, then back to Mirror B and then onto the Receiver R.
The path length (measured on the equipment) is 400ft.
Note: The light strikes mirror Z at a closing velocity of 100ft/sec, because mirror Z is moving away at 50ft/sec. The light reflection loses another 50ft/sec. because the mirror is still moving away at 50ft/sec.
The speed of the light reflection relative to mirror Z is 100ft/sec. Confusing isn’t it? But it is true. Cricketers, table tennis players, etcetera all use this fact whilst playing their games, and without a honours degree in physics or engineering. Although not an everyday problem in mechanics it does arise quite often in machinery design.
TD(E>mZ) = Time taken from Emitter E to Mirror Z.
= TD(E>mZ)/V2(E>mZ) = 300/150 = 2 sec.
DT(mZ>mA) = Time taken from Mirror Z to Mirror A.
= TD(mZ>mA)/V(mZ>mA) = 50/50 = 1 sec.
T(mZ>R) = Time taken from Mirror Z to Mirror A.
= T(mZ>R)/V(mZ>R) = 111.8/111.8 = 1 sec.
—————————————————
Notes on lack of time differences in the Michelson – Morley experiment.
If you place the experiment on a train and rotate it through 360° and measure the time taken for the light to travel from the emitter to the the receiver at various angular positions, no time differences will be detected.
——————————————–
If you are in a car travelling eastwards towards the rising Sun at a road speed of 100 mph, then your speed relative to the Sun (It being a ‘fixed point in the universe relative to us), would be approximately 1,100 mph. (The earth rotates at approximately 1000 mph.)
If later the same day on the same road and still travelling at 100 mph, but travelling in a westerly direction towards the setting sun, your speed relative to the sun would be approximately minus 900 mph. Therefore your speed relative to the sun would have changed by 2000 mph, (i.e. from +1100mph to -900mph.) in the 12 hours between the two journeys.
——————————-
If you replace the car with a beam of light and carry out similar timings the light will actually be travelling at c + 1000 mph when travelling towards the sunrise, and c-1000mph when travelling towards the sunset, ( c = The claimed constant speed of light.) The velocity of the light (Relative to the Sun) changes by 2000mph between these
Let us change our apparatus to a tube 300,000 kilometres long (approximately). Inside this tube there is a light transmitter(A) at one end and a light receiver(B) at the other end. We set this tube travelling towards the sun, with end B at the leading end, at a speed of 300,000 kps. When B is at a distance of 300,000 kilometres from the sun we fire the light emmitter at A, towards B (the receiver). One second later end B hits the sun at exactly the same time as the light hits (B). At the time (A) is triggered it is 600,000 kilometres away from the sun.
During this one second the light has travelled 600,000 kilometres and this light has travelled at twice the speed of light!
If we had reversed the tube so that A was at the front end and carried out the same procedure, the light would travel from A to B in two seconds. (end B is still 300,000 kilometres from the sun when end A hits the sun). The light still travels the length of the tube in one second but to an outside observer the light front would be static whilst the end B would be travelling at 300,000 kps towards the light front.
It is this aspect of the Michelson – Morley Experiment that confuses the physicists (plus one or two trigonometry mistakes.)
The man plus the train, the car plus the road, are just different versions of the Michelson – Morley experiment.
Author – Brian Williams
-
The Mechanics of True Perspective.
Posted on July 25th, 2014 No commentsINTRODUCTION.
Throughout known history man has produced pictorial representations of his environment, from the earliest cave paintings of animals, hunting scenes, weapons, & man, through to the masterpieces of the great painters of the renaissance. Bearing in mind the wide variations in artistic talents of the general population of today, and considering the extremely low population of early man, one is tempted to conclude that early man had a greater artistic capability. Many cave painting have great technical assurance, and considering the severe circumstances in which they were produced, would indicate an enthusiasm beyond that of most artists. Produced on rough rock faces, using charcoal or home-made dyes, applied with sticks, fingers etc., carried out in semi-darkness by the light of guttering torches, and in an atmosphere full of smoke, it is doubtful that many of our great painters could have done better in the same circumstances.
If these same cave painters had sable brushes, stretched canvases, and the wide variety of painting media that we have today, they would be world famous artists. It is sad to consider that their greatest works would have been done outside the caves, in more congenial circumstances, but these would have been destroyed by weather and erosion.
It is clear that these cave painters understood the importance of perspective, demonstrated in many of the their paintings. Even the artists of ancient Egypt, often unjustly accused of not being able to ‘draw properly’ (See later note.), understood the basic requirements of perspective.
The technical aspects of perspective were intensively studied during the renaissance, and in fact our present systems of perspective show no improvement on these early studies.
I first became interested in perspective whilst a student at Bolton Municipal College of Art, where, having a keen interest in mechanics as well as art, I was intrigued by the apparent incompatibility between the art of perspective and the mechanics of perspective as given in all the text books on the subject.
One of the points that emerged from my later research was the practical incompatibility of the art of perspective, with the mechanics of perspective. This will become apparent as we continue.
A further point relates to what in many cases are referred to as ‘ optical illusions’, or faults in our eye or brain. In fact, many of these are accurate assessments of the information (in the form of light) that is arriving at the eye.
Note: – The stylised ‘side only’ drawings on most Egyptian artefacts were certainly not created due to any lack of ability to draw full frontal or angled drawings. The artists’ abilities are clearly demonstrated by the large number of superb statues, masks etc. in existence. There are also full frontal drawings in existence that were patterns for statues. There are various possible reasons for the stylised form of art;
a. Economy. It is easier to draw figures in this way, and the large numbers of figures in most tombs etc. would made this an economic method of drawing.
b. Suitability. Most figures are carrying out some form of activity. It is easier to show these activities in a side view rather than a full frontal or angled view. This style of drawing figures is often used in instruction manuals today.
c. Labour. It is unlikely that the master artist carried out all the paintings, therefore numerous other artists or semi-skilled technicians would have carried out the work. ‘Patterns’ would be used for guidance by these ‘auxiliary’ artists.
d. Style. More realistic drawings methods would lose much of the important aspect of the drawings, i.e. they are primarily a means of recording messages. Every picture has clarity of meaning, even if the picture is not truly realistic.
——————————————————
True Perspective.
The simple drawing below is of a group of buildings. This is actually what you see, even though you will initially argue against my statement.
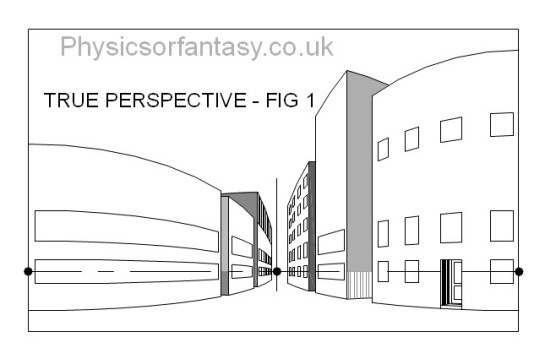
Let us look at Fig.2 which shows four areas to consider.
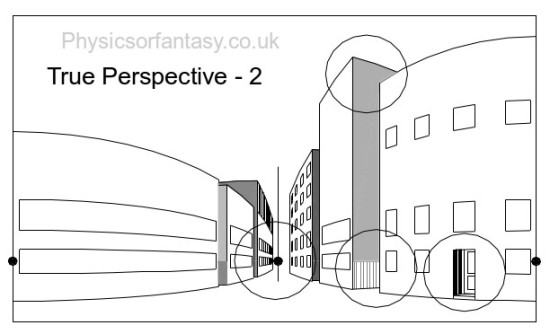
Let us now look at these areas in isolation.
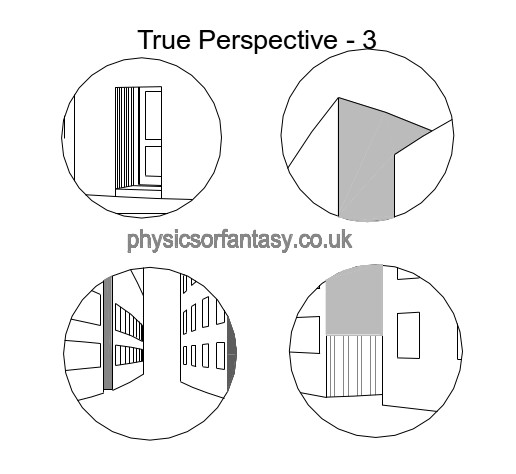
Looked at in isolation, as happens with the human eye’s small area of high definition, I think you will agree that they do not seem to be particularly distorted.
Normal Methods used in Perspective.
Author – Brian Williams
Much more to come on this subject.
-
Retinal Haemorrhages.
Posted on April 25th, 2012 No commentsNOTE:- All names and dates have been changed apart from in extracts from medical literature.
RETINAL HAEMORRHAGES.
Consider this extract from ‘CLINICAL EXAMINATION’, page 384. “ The use of the ophthalmoscope is dealt with in Chapter 11. The difficulties of this examination in children are often considerable. To maintain the young child’s gaze in a fixed direction it is usually necessary for a second person to arrange some diversion which for the child has an element of expectancy about it. The examiner, after positioning the patient may say ,’Tell me when the torch flashes’, the torch being held in an appropriate position by a helper who may be mother or nurse, or ‘Tell me how many fingers nurse has up – how many now?,’ the nurse changing the numbers of fingers and thus maintaining the child’s attention. With younger children sedation may be necessary and in certain circumstances even a general anaesthetic may be required.
Extract from Medical Literature.
“They [Retinal haemorrages] are also reported to occur in a percentage of new born infants e.g. 14% in a study by Sezen:(……) and 20% Small(…) who found evidence of retinal haemorrhages but no evidence of intracranial injury on Magnetic Resonance Scanning subsequently. These retinal haemorrhages resolve within a short time after birth e.g. 10 days…….”.
Here is an argument stating that there is evidence of ‘spontaneous’ retinal haemorrhages’ in a significant number of the newly born, but that these normally clear in 10 days.
It is evident from the above extract that retinal haemorrhages are commonly caused by extremely minor ‘trauma’ and are not particularly indicative of a shaking injury.
One should remember that blood circulates by surges in pressure. Restriction of the veins leading away from any part of the body cause excessive pressure surges in that part of the body.
Place an elastic band on a finger or around your wrist and this excessive pressure can be clearly felt in the extremity. This is because the elastic band only restricts the veins carrying blood away from the affected part, but the arterial blood flowing into the hand or finger is not restricted. If the restriction is long lasting then serious damage can be caused to that part of the body. If one considers a blood pressure cuff being gradually inflated over a period of weeks or months, the pressure in the veins will gradually build up until the pressure exerted by the cuff is sufficient to stop the blood flowing. If then left in this state serious damage would be caused.
In regard to retinal haemorrhages, if the veins leading from the eye are restricted, as can happen in hydrocephalus, there can be either a rapid or a gradual reduction in the blood flow. As the veins themselves require blood to sustain themselves in good working order, lack of blood flow weakens them. The areas of the eye being served by the capillaries are also damaged due to lack of blood flow. The increased pressure pulses remain but no fresh blood is being supplied. After a while the weakest veins, i.e. the capillaries in the eye, or the part being supplied, break down due to the increased pressure pulses and the poor condition.
It is evident therefore that there can be no precise time scale for the development of retinal haemorrhages.
My Own Retinal Haemorrhage.
The year following this court case I suffered a retinal haemorrhage.
I was driving out of a city centre when a black spot appeared in my right eye. This quickly changed into a black snakelike shape and then into multiple treelike branches, and finally a deep red haze that completely closed all vision in my right eye.
Luckily, having worked on the above case I immediately understood what had happened. I asked a passer-by for the location of a doctor, and within 20 minutes I was on my way to hospital by ambulance. A week later I had the first laser treatment.
Discussing the problem with the surgeon, she said that I must have an infection or I must have had a bang to my head in the last couple of days. Tests showed no signs of infections, and I certainly had no bangs to my head in the last few days.
However, I did have a severe bang to my head 6 weeks before, when I bent down to stroke our labrador dog who jumped up to lick me at the same time, causing a collision of heads. Both of us ended up on the floor stunned for a few minutes.
For the weeks following, from dusk onwards I was aware of flashing lights in front of my right eye. These continued for the 6 weeks until the retinal haemorrhage occured. I went into hospital for laser treatment for the retinal haemorrhage about a week to 10 days later.
The surgeon was adamant that the dog incident was too early to have caused my retinal haemorrhage, but could not suggest an alternative hypothesis.
Author – Brian Williams
-
Physics in the News – Speed of Light.
Posted on October 3rd, 2011 No commentsYou are on a train travelling Eastwards at the equator at 100 miles per hour relative to the Earth’s surface. The Earth’s surface is also travelling Eastwards at approximately 1000 miles per hour. If you walk from the rear of the train towards the front of the train at a speed of 5 miles per hour, your combined speed would be 1,105 miles per hour. This is not your actual true speed because we still have to add in the speed of the Earth around the Sun. We would also have to add in the speed of the solar system within the galaxy. We would also need to add in the speed of galaxy relative to the known universe. We would also need to add in the speed of the known universe, relative to a fixed point in space. We can never know the location of a fixed point in space.
It is clear that it is impossible (even for an engineer) to work out what your actual true speed is.
This same problem applies to the speed of light. However , physicists still argue that the actual true speed is constant at approximately 300,000 kilometres per second. This is based on Einstein’s ludicrous interpretation of the Michelson -Morley experiment.
This was a simple mechanical assembly fitted with lights and mirrors. Unfortunately, Michelson, Morley and Einstein could not understand the mechanics of this apparatus. Not understanding the mechanics they were confused when they did not get the results that they expected. The actual results obtained were exactly those expected (by engineers) from the mechanics of the apparatus.
This confusion is still with us today, and virtually all of modern physics is affected by Einstein’s interpretation of this experiment. It also explains why none of the hypotheses of the physics establishment have ever managed to cross the boundary between hypothesis and theory. {A Hypothesis can be any silly idea, to become a Theory it must be demonstrated to satisfy all known facts relating to the subject.]
Brian Williams
Author
For more details see http://www.physicsorfantasy.co.uk/?page_id=6
-
Facts and Fallacies about Modern Physics – 2
Posted on July 19th, 2011 No comments- Einstein did not invent the atom bomb. See Einstein and the Bomb.
- Physicists have not discovered dozens of sub-atomic particles. In fact they haven’t even discovered one. Even the electron is only at the ‘theory’ stage, but there is reasonable circumstantial evidence for its existence. See How Physicists “Find” their Particles.
- The speed of light is not a constant. See Physics or Fantasy – Section 1. Michelson – Morley made some basic errors in their maths (simple trigonometry). Einstein and the physics establishment accepted the maths without checking them, and arrived at the constant speed of light mythology.
- There is no such thing as the ‘wavelength of light’. Waves can be ‘created’ in streams of light just as they can be ‘created’ in streams of water, but you would look silly if you started talking about the ‘wavelength’ of water. ‘Waves’ in light are created by the apparatus itself. In fact all waves in anything you consider are either ‘created’, i.e. something other than the material or fluid itself actually causes the waves, or are ‘generated’ as in Radio and television waves. or just like alternating power supplies. If you hold a stick in a flowing stream of water, waves are ‘created’ around the stick. The ripples (Waves) in the sand are ‘created’ by the rush of water up the beach and have nothing to do with the waves in the water itself. The sand ‘waves’ in deserts are created by the wind and do not indicate that wind or sand are part of the electromagnetic spectrum. Physicists just do not understand the nature of waves because it is mechanics.
- Blue light does not have more energy than other colours, in fact it has the lowest energy level. See the posts on colour.
- No-one has ever seen an electron. No-one has ever seen an atom. However, we see by the quantity and speed of electrons entering our eyes. Yes, I do mean electrons, not Photons, there is no requirement for the hypothetical photon particle to explain vision.
I will add more as I think of them.
-
Physics in the News – Two Slit Interferometer.
Posted on June 4th, 2011 No commentsFrom BBC News – 3 June 2011
Quantum mechanics rule ‘bent’ in classic experiment
By Jason Palmer Science and technology reporter, BBC News
 Light can interfere with itself just as water ripples can add to or cancel one another
Light can interfere with itself just as water ripples can add to or cancel one anotherResearchers have bent one of the most basic rules of quantum mechanics, a counterintuitive branch of physics that deals with atomic-scale interactions.
Its “complementarity” rule asserts that it is impossible to observe light behaving as both a wave and a particle, though it is strictly both.
In an experiment reported in Science, researchers have now done exactly that.
They say the feat “pulls back the veil” on quantum reality in a way that was thought to be prohibited by theory.
Quantum mechanics has spawned and continues to fuel spirited debates about the nature of what we can see and measure, and what nature keeps hidden – debates that often straddle the divide between the physical and the philosophical.
For instance, a well-known rule called the Heisenberg uncertainty principle maintains that for some pairs of measurements, high precision in one necessarily reduces the precision that can be achieved in the other. [ This is not so. Brian]
One embodiment of this idea lies in a “two-slit interferometer”, in which light can pass through one of two slits and is viewed on a screen.
Let a number of the units of light called photons through the slits, and an interference pattern develops, like waves overlapping in a pond. However, keeping a close eye on which photons went through which slits – what may be termed a “strong measurement” – destroys the pattern. Etcetera, etcetera.
———————————————-
More waffle from the physics establishment. This experimental information is covered in my book Physics or Fantasy – Section 1 – Light and Relativity.
The experimental results obtained show that light functions strictly in accordance with normal fluid mechanics, and have nothing to do with quantum mechanics. Just as in my experiments on colour, the main items of equipment in these experiments are the slits themselves. The significance of the ‘mechanics’ of the slits is totally ignored by the physicists, mainly due to their creed that mechanics cannot have anything to do with physics.
Light is the ultimate fluid. People do not think of light as a fluid, yet it functions like a fluid. The main difficulty is that light is so fluid it that it travels a lot faster than liquids or gases. The main definition of a fluid is it takes the form of any shape that contains it.
The speed at which a fluid conforms to the shape of the containing vessel determines its fluidity. Treacle, Oil, Water, Dry Sand, Oxygen, Hydrogen etcetera are all fluids. However, they all take different amounts of time to fill any containment of them. They are also subject to to gravity and internal frictional forces. Light is only very, very little affected by gravity, and is only affected by external friction, again, only in a very small way. The slit experiments demonstrate the effects of friction on light.
However, do not expect to put light into a bottle, put a stopper in and use it later. Once light is stopped, its energy is dissipated. The particles (Or if you are a physicist, wavy lines) are still there but have lost their energy. The particles have to have a certain speed to be detected by the eye as ‘light’. Below this speed they cannot trigger the eye/brain receptors.
The experiments are explained in simple language.
See also
-
MALICIOUS ATTACK ON THIS WEB SITE
Posted on May 12th, 2011 No commentsTHERE IS AN ATTACK ON THIS WEB SITE. SOMEONE IS COPYING MY POSTS ONTO THEIR OWN SITE AND SPAMMING IT AROUND THE WORLD.
THE FAKE WEB SITE IS APPARENTLY BEING LOADED WITH ADVERTISING FOR WHICH THEY ARE OBVIOUSLY BEING PAID. SO FAR ALL THE ADVERTS SEEM TO BE VIA GOOGLE, BUT MAY BE EXTENDED TO OTHERS.
IF YOU RECEIVE ONE OF THESE PLEASE ADVISE ME AND ALSO ADVISE GOOGLE OR ANY OF THE ADVERTISERS. GOOGLE HAVE BEEN TOLD BY ME OF THESE ATTACKS.
GET THE URL NUMBER AND ANY OTHER DATA THAT YOU CAN.
YOUR OWN SITE COULD BE TARGETED NEXT.
The Advertisers must know who they are paying.
————————–
THERE ARE NO ADVERTS ON MY WEBSITE APART FROM THAT RELATING TO MY BOOK.
THERE ARE NO VIDEOs, AND THERE IS UNLIKELY TO BE MANY SPELLING MISTAKES, ITEMS REFERRED TO RELATING TO FAKE SITES.
———-
THE ONLY TIME I SEND UNREQUESTED COPIES OF MY POSTS, IT IS TO PUBLIC BODIES SUCH AS COLLEGES AND UNIVERSITIES
-
The Difference Between Reflected Light and Projected Light
Posted on March 16th, 2011 No commentsReflected light is composed of a certain percentage of each true primary colour (Yellow, Red and Dark Blue). If we consider a mid Green consisting of 50% Blue and 50% Yellow, the reflected energy is much less than the same area having all Yellow. It is also much more than the same area having all Blue.
If you think of any pigment as a number of very small tiles each having its own colour, then if you only have Yellow tiles and Blue tiles to play with, your overall possible perceived colours are limited to all Yellow, all Blue or limited shades of Green. The maximum energy emitted from any area of the pigment would be if only Yellow tiles were used.
If you have some White tiles you can have a lighter Yellow, a lighter Blue or an extended range of Greens i.e some lighter.
If you have some Black tiles you can have a darker Yellow, a darker Blue or an extended range of Greens i.e some darker.
With projected light it is more more complex. There are two different situations with projected light.
Type 1. If you have source of ‘true’ Yellow light that shines through a series of small square holes separated by similar sized small Blue filters., the resulting colour will be a light Green. This is because some of the Yellow light will pass through the Blue filter producing a dark Green. This dark Green, when added to the Yellow will create the light Green. In comparison with the coloured tiles in which we had Yellow and Blue tiles, we now have, in effect, Yellow and dark Green tiles. {See Colour Filters}
Type 2. See Projected Light.
Author – Brian Williams.